Abstract
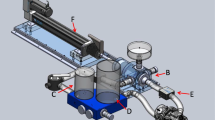
We sought to build a robust pulsatile flow model permitting comparison of 3D Color Doppler and phase contrast magnetic resonance (PCMR) flow and orifice area measurements of prosthetic valve function that would provide an in vitro gold standard for evaluation of the accuracy of new imaging techniques. A multi-modality compatible, pulsatile linear actuation system, control software, and mock circulation loop was designed and created using non-ferromagnetic components, NI Labview, and rapid prototyping. The developed system’s ability to create pulsatile, controlled flow was assessed by examining peak diastolic flow, diastolic period, and diastolic flow volume (DFV) of 12 consecutive cardiac cycles at 3 different DFVs. Reproducibility of flow was tested by examining the systems ability to generate the same target DFV after draining and re-configuring the flow loop components. Furthermore, the system was evaluated by CMR and echocardiography to examine image quality, DFV quantification, and an initial functional assessment of prosthetic mitral valve (MV) orifice area by four different imaging methods. The system was able to reproducibly create prescribed flow volumes across 31 mm bioprosthetic and mechanical MV’s under a range of flow conditions. In vitro image quality for the identification of MV sewing ring, struts and leaflets was similar to clinical imaging quality for both echocardiographic and MRI methods. For the quantification of DFV, the PCMR method demonstrated an error range of 1.4–9.2% and a mean difference of 4 ± 2 mL/beat compared to the flowmeter reference standard. Across the three flow conditions the bioprosthetic MV diastolic flow area was statistically similar by MRI planimetry, phase contrast MRI planimetry, and 3D color Doppler planimetry methods (mean area 3.2 ± 0.5 cm2, ANOVA, p = NS), but the effective orifice area by 2D spectral Doppler (continuity) method was smaller (mean area 2.0 ± 0.3 cm2, ANOVA, p < 0.001). We describe the development and initial functional testing of a novel MRI-compatible flow loop system for comparison of multimodality imaging techniques to assess prosthetic valve function.











Similar content being viewed by others
References
Blauwet, L. A., J. F. Malouf, H. M. Connolly, D. O. Hodge, K. N. Evans, R. M. Herges, T. M. Sundt, III, and F. A. Miller, Jr. Comprehensive echocardiographic assessment of normal mitral Medtronic Hancock II, Medtronic Mosaic, and Carpentier-Edwards Perimount bioprostheses early after implantation. J. Am. Soc. Echocardiogr. 23:656–666, 2010.
Bonow, R. O., B. A. Carabello, K. Chatterjee, A. De Leon, Jr, D. P. Faxon, M. D. Freed, W. H. Gaasch, B. W. Lytle, R. A. Nishimura, P. T. O’Gara, R. A. O’Rourke, C. M. Otto, P. M. Shah, J. S. Shanewise, S. C. Smith, Jr, A. K. Jacobs, C. D. Adams, J. L. Anderson, E. M. Antman, V. Fuster, J. L. Halperin, L. F. Hiratzka, S. A. Hunt, B. W. Lytle, R. Nishimura, R. L. Page, and B. Riegel. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 48:e1-148, 2006.
Garver, D., R. G. Kaczmarek, B. G. Silverman, T. P. Gross, and P. M. Hamilton. The epidemiology of prosthetic heart valves in the United States. Tex. Heart Inst. J. 22:86–91, 1995.
Hasenkam, J. M., S. Ringgaard, K. Houlind, R. M. Botnar, H. Stodkilde-Jorgensen, P. Boesiger, and E. M. Pedersen. Prosthetic heart valve evaluation by magnetic resonance imaging. Eur. J. Cardiothorac. Surg. 16:300–305, 1999.
Hendel, R. C., M. R. Patel, C. M. Kramer, M. Poon, R. C. Hendel, J. C. Carr, N. A. Gerstad, L. D. Gillam, J. M. Hodgson, R. J. Kim, C. M. Kramer, J. R. Lesser, E. T. Martin, J. V. Messer, R. F. Redberg, G. D. Rubin, J. S. Rumsfeld, A. J. Taylor, W. G. Weigold, P. K. Woodard, R. G. Brindis, R. C. Hendel, P. S. Douglas, E. D. Peterson, M. J. Wolk, J. M. Allen, and M. R. Patel. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J. Am. Coll. Cardiol. 48:1475–1497, 2006.
Krim, S. R., R. P. Vivo, A. Patel, J. Xu, S. R. Igo, W. A. Zoghbi, and S. H. Little. Direct assessment of normal mechanical mitral valve orifice area by real-time 3D echocardiography. JACC Cardiovasc. Imaging 5:478–483, 2012.
Kvitting, J. P., P. Dyverfeldt, A. Sigfridsson, S. Franzen, L. Wigstrom, A. F. Bolger, and T. Ebbers. In vitro assessment of flow patterns and turbulence intensity in prosthetic heart valves using generalized phase-contrast MRI. J. Magn. Reson. Imaging 31:1075–1080, 2010.
Li, C. P., S. F. Chen, C. W. Lo, and P. C. Lu. Turbulence characteristics downstream of a new trileaflet mechanical heart valve. ASAIO J. 57:188–196, 2011.
Little, S. H., S. R. Igo, B. Pirat, M. McCulloch, C. J. Hartley, Y. Nose, and W. A. Zoghbi. In vitro validation of real-time three-dimensional color Doppler echocardiography for direct measurement of proximal isovelocity surface area in mitral regurgitation. Am. J. Cardiol. 99:1440–1447, 2007.
Little, S. H., B. Pirat, R. Kumar, S. R. Igo, M. McCulloch, C. J. Hartley, J. Xu, and W. A. Zoghbi. Three-dimensional color Doppler echocardiography for direct measurement of vena contracta area in mitral regurgitation: in vitro validation and clinical experience. JACC Cardiovasc. Imaging 1:695–704, 2008.
Pham, N., H. Zaitoun, T. L. Mohammed, E. LaPena-Almaguer, F. Martinez, G. M. Novaro, and J. Kirsch. Complications of aortic valve surgery: manifestations at CT and MR imaging. Radiographics 32:1873–1892, 2012.
Pibarot, P., and J. G. Dumesnil. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart 92:1022–1029, 2006.
Quail, M. A., J. Nordmeyer, S. Schievano, M. Reinthaler, M. J. Mullen, and A. M. Taylor. Use of cardiovascular magnetic resonance imaging for TAVR assessment in patients with bioprosthetic aortic valves: comparison with computed tomography. Eur. J. Radiol. 81:3912–3917, 2012.
Rabbah, J. P., N. Saikrishnan, and A. P. Yoganathan. A novel left heart simulator for the multi-modality characterization of native mitral valve geometry and fluid mechanics. Ann. Biomed. Eng. 41:305–315, 2013.
Sugeng, L., S. K. Shernan, L. Weinert, D. Shook, J. Raman, V. Jeevanandam, F. DuPont, J. Fox, V. Mor-Avi, and R. M. Lang. Real-time three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J. Am. Soc. Echocardiogr. 21:1347–1354, 2008.
Valdes-Cruz, L. M., A. P. Yoganathan, T. Tamura, F. Tomizuka, Y. R. Woo, and D. J. Sahn. Studies in vitro of the relationship between ultrasound and laser Doppler velocemitry and applicability to the simplified Bernoulli relationship. Circulation 73(2):300–308, 1986.
Vasko, S. D., S. J. Goldberg, J. A. Requarth, and H. D. Allen. Factors affecting accuracy of in vitro valvar pressure gradient estimates by Doppler ultrasound. Am. J. Cardiol. 54(7):893–896, 1984.
von Knobelsdorff-Brenkenhoff, F., R. Rottgen, and J. Schulz-Menger. Complementary assessment of aortic bioprosthetic dysfunction using cardiac magnetic resonance imaging and computed tomography. J. Heart Valve Dis. 21:20–22, 2012.
von Knobelsdorff-Brenkenhoff, F., A. Rudolph, R. Wassmuth, S. Bohl, E. E. Buschmann, H. Abdel-Aty, R. Dietz, and J. Schulz-Menger. Feasibility of cardiovascular magnetic resonance to assess the orifice area of aortic bioprostheses. Circ. Cardiovasc. Imaging 2:397–404, 2009.
von Knobelsdorff-Brenkenhoff, F., A. Rudolph, R. Wassmuth, and J. Schulz-Menger. Assessment of mitral bioprostheses using cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 12:36, 2010.
Yap, C. H., H. S. Kim, K. Balachandran, M. Weiler, R. Haj-Ali, and A. P. Yoganathan. Dynamic deformation characteristics of porcine aortic valve leaflet under normal and hypertensive conditions. Am. J. Physiol. Heart Circ. Physiol. 298:H395–H405, 2010.
Yap, C. H., N. Saikrishnan, G. Tamilselvan, and A. P. Yoganathan. Experimental technique of measuring dynamic fluid shear stress on the aortic surface of the aortic valve leaflet. J. Biomech. Eng. 133:061007, 2011.
Acknowledgments
This work is funded in part by Grant support from the American Heart Association Beginning Grant-in-Aid (MJ, SL), and product support from Medtronic Inc. The collaboration between Houston Methodist DeBakey Heart & Vascular Center and ExxonMobil Upstream Research Company was developed through the Pumps & Pipes annual symposium (pumpsandpipes.com), which aims to foster research opportunities between the Medical, Energy, and Aerospace industries of Houston.
Conflict of Interest
Matthew S. Jackson, Stephen R. Igo, Thomas E. Lindsey, Dimitrios Maragiannis, Karen E. Chin, Kyle Autry, Robert Schutt III, Dipan J., Pietro Valsecchi, and William B. Kline declare that they have no conflict of interest. Stephen H. Little has received a research Grant Number #11BGIA5840008 from the American Heart Association
Human Subjects
No human studies were carried out by the authors for this article
Animal Studies
No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Jackson, M.S., Igo, S.R., Lindsey, T.E. et al. Development of a Multi-modality Compatible Flow Loop System for the Functional Assessment of Mitral Valve Prostheses. Cardiovasc Eng Tech 5, 13–24 (2014). https://doi.org/10.1007/s13239-014-0177-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-014-0177-7