Abstract
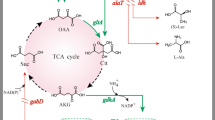
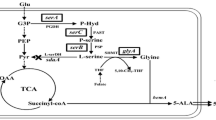
The direct fermentative production of l-serine from renewable biomass using Corynebacterium glutamicum is attracting increasing attention. In this study, wild-type C. glutamicum SYPS-062 produced up to 6.65 ± 0.23 g/L l-serine; to further improve l-serine production, the serA gene was cloned, and the C-terminal domain of 3-phosphoglycerate dehydrogenase (PGDH) from this strain was truncated. When expressed in Escherichia coli, the resultant mutein SerAΔ197 showed a specific PGDH activity of 1.092 ± 0.05 U/mg protein, representing a decrease of 25.87 % from that encoded by serA, and was no longer sensitive to high concentrations of l-serine. When serA Δ591 was overexpressed in C. glutamicum SYPS-062, the activity of PGDH in C. glutamicum pJC1-tac-serA Δ591 increased by 47.72 %, and the resultant strain C. glutamicum pJC1-tac-serA Δ591 could accumulate 7.69 ± 0.22 g/L l-serine. Furthermore, when serA Δ591 was overexpressed in C. glutamicum SYPS-062ΔsdaA, the resultant strain could accumulate 8.84 ± 0.23 g/L l-serine at 102 h, and the yield of l-serine on cells (Y p/x) improved by 60 % when compared with that noted in the control. These results demonstrate that l-serine production in C. glutamicum SYPS-062 could be improved by overexpressing a C-terminal truncation of PGDH in combination with other genetic modifications.



Similar content being viewed by others
References
Eggeling L, Bott M (eds) (2005) Handbook of Corynebacterium glutamicum. CRC, Boca Raton
Eggeling L, Sahm H (1999) l-Glutamate and l-lysine: traditional products with impetuous developments. Appl Microbiol Biotechnol 52:146–153
Ema M, Kakimoto T, Chibata I (1979) Production of l-serine by Sarcina albida. Appl Environ Microbiol 37:1053–1058
Grant GA (1989) A new family of 2-hydroxyacid dehydrogenases. Biochem Biophys Res Commun 165:1371–1374
Grant GA, Schuller DJ, Banaszak LJ (1996) A model for the regulation of d-3-phosphoglycerate dehydrogenase, a Vmax-type allosteric enzyme. Protein Sci 5:34–41
Hagishita T, Yoshida T, Izumi Y, Mitsunaga T (1996) Efficient L-serine production from methanol and glycine by resting cells of Methylobacterium sp. strain MN43. Biosci Biotechnol Biochem 60:1604–1607
Ho CL, Noji M, Saito M, Saito K (1999) Regulation of serine biosynthesis in Arabidopsis. Crucial role of plastidic 3-phosphoglycerate dehydrogenase in non-photosynthetic tissues. J Biol Chem 274:397–402
Hsiao HY, Wei T (1986) Enzymatic production of l-serine with a feedback control system for formaldehyde addition. Biotechnol Bioeng 28:1510–1518
Izumi Y, Yoshida T, Miyazaki SS, Mitsunaga T, Ohshiro T, Shimao M, Miyata A, Tanabe T (1993) l-Serine production by a methylotroph and its related enzymes. Appl Microbiol Biotechnol 39:427–432
Lai SJ, Zhang Y, Liu SW, Liang Y, Shang XL, Chai X, Wen TY (2012) Metabolic engineering and flux analysis of Corynebacterium glutamicum for l-serine production. Sci China Life Sci 55:283–290
Li Y, Chen GK, Tong XW, Zhang HT, Liu XG, Liu YH, Lu FP (2012) Construction of Escherichia coli strains producing l-serine from glucose. Biotechnol Lett 34:1525–1530
Marx A, de Graaf AA, Wiechert W, Eggeling L, Sahm H (1996) Determination of the fluxes in the central metabolism of Corynebacterium glutamicum by nuclear magnetic resonance spectroscopy combined with metabolite balancing. Biotechnol Bioeng 49:111–129
Netzer R, Peters-Wendisch P, Eggeling L, Sahm H (2004) Cometabolism of a nongrowth substrate: l-serine utilization by Corynebacterium glutamicum. Appl Environ Microb 70:7148–7155
Omori K, Kakimoto T, Chibata I (1983) l-Serine production by a mutant of Sarcina albida defective in l-serine degradation. Appl Environ Microbiol 45:1722–1726
Peters-Wendisch P, Netzer R, Eggeling L, Sahm H (2002) 3-phosphoglycerate dehydrogenase from Corynebacterium glutamicum: the C-terminal domain is not essential for activity but is required for inhibition by l-serine. Appl Microbiol Biotechnol 60:437–441
Peters-Wendisch P, Stolz M, Etterich H, Kennerknecht N, Sahm H, Eggeling L (2005) Metabolic engineering of Corynebacterium glutamicum for l-serine production. Appl Environ Microbiol 71:7139–7144
Saski R, Pizer LI (1975) Regulatory properties of purified 3-phosphoglycerate dehydrogenase from Bacillus subtilis. Eur J Biochem 51:415–427
Sauer U, Eikmanns BJ (2005) The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev 29:765–794
Schuller DJ, Grant GA, Banaszak LJ (1995) The allosteric ligand site in the Vmax-type cooperative enzyme phosphoglycerate dehydrogenase. Nat Struct Biol 2:69–76
Schweitzer JE, Stolz M, Diesveld R, Etterich H, Eggeling L (2009) The serine hydroxymethyltransferase gene glyA in Corynebacterium glutamicum is controlled by GlyR. J Biotechnol 139:214–221
Stauffer GV (1996) Biosynthesis of serine, glycine, and one-carbon units, 2nd edn. ASM, Washington, DC
Vallino JJ, Stephanopoulos G (1994) Carbon flux distributions at the pyruvate branch point in Corynebacterium glutamicum during lysine overproduction. Biotechnol Prog 10:320–326
Zhang X, Dou W, Xu H, Xu Z (2010) Metabolic flux analysis of l-serine synthesis by Corynebacterium glutamicum SYPS-062. Chinese J Biotechol 26:1363–1371
Zhao G, Winkler ME (1996) A novel alpha-ketoglutarate reductase activity of the serA-encoded 3-phosphoglycerate dehydrogenase of Escherichia coli K-12 and its possible implications for human 2-hydroxyglutaric aciduria. J Bacteriol 178:232–239
Acknowledgments
This work was supported financially by the High Tech Development Program of China (863 Project, No. 2012AA022102) and the production project of Ministry of Education of Guangdong province (No.2012B091000083).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, G., Jin, X., Guo, W. et al. Characterization, modification, and overexpression of 3-phosphoglycerate dehydrogenase in Corynebacterium glutamicum for enhancing l-serine production. Ann Microbiol 65, 929–935 (2015). https://doi.org/10.1007/s13213-014-0936-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-014-0936-6