Abstract
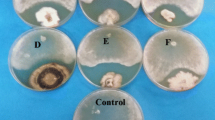
Sclerodermatoid fungi basidiomes were collected from northern Thailand and pure cultures were isolated. The morphology and molecular characteristics identified them as Astraeus odoratus, Phlebopus portentosus, Pisolithus albus and Scleroderma sinnamariense. This study investigated the in vitro ability of selected fungi to produce indole-3-acetic acid (IAA), to solubilize different toxic metal (Co, Cd, Cu, Pb, Zn)-containing minerals, and metal tolerance. The results indicated that all fungi are able to produce IAA in liquid medium. The optimum temperature for IAA production of all fungi was 30 °C, and the optimum concentration of L-tryptophan of Astraeus odoratus, Pisolithus albus and Scleroderma sinnamariense was 2 mg ml−1. The highest IAA yield (65.29 ± 1.17 μg ml−1) was obtained from Phlebopus portentosus after 40 days of cultivation in culture medium supplemented with 4 mg ml−1 of L-tryptophan. The biological activity tests of fungal IAA showed that it can simulate coleoptile elongation, and increase seed germination and root length of tested plants. In addition, the metal tolerance and solubilizing activities varied for different minerals and fungal species. The presence of metal minerals affected fungal growth, and cobalt carbonate showed the highest toxicity. The solubilization index decreased when the concentration of metal minerals increased. Astraeus odoratus showed the lowest tolerance to metals. This is the first report of in vitro IAA production, solubilization of insoluble metal minerals and metal tolerance abilities of the tested fungi.






Similar content being viewed by others
References
Ahemad M, Khan MS (2010) Growth promotion and protection of lentil (Lens esculenta) againt herbicide stress by Rhizobium species. Ann Microbiol 60:735–745
Ahemad M, Khan MS (2012) Evaluation of plant-growth-promoting activities of rhizobacterium Pseudomonas putida under herbicide stress. Ann Microbiol 62:1531–1540
Ahmad F, Ahmad I, Khan MS (2005) Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk J Biol 29:29–34
Apine OA, Jadhav JP (2011) Optimization of medium for indole-3-acetic acid production using Pantoea agglomerans strain PVM. J Appl Microbiol 110:1235–1244
Barker SJ, Tagu D (2000) The roles of auxins and cytokinins in mycorrhizal symbioses. J Plant Growth Regul 19:144–154
Binder M, Hibbett DS (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98:971–981
Blaudez D, Jacob C, Turnau K, Colpaert JV, Ahonen-jonnarth U, Finlay R, Botton B, Chalot M (2000) Differential responses of ectomycorrhizal fungi to heavy metals in vitro. Mycol Res 104:1366–1371
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with mycorrhizas in forestry and agriculture. ACIAR Monograph, Camberra
Chaiharn M, Lumyong L (2011) Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr Microbiol 62:173–181
Chung HC, Kim DH, Lee SS (2002) Mycorrhizal formations and seedling growth of Pinus densiflora by in vitro synthesis with the inoculations of ectomycorrhizal fungi. Mycobiology 30:70–75
Chung KR, Shilts T, Ertürk, Timmer LW, Ueng PP (2003) Indole derivatives produced by the fungus Colletotrichum acutatum causing lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol Lett 266:23–30
Chung KR, Tzeng DD (2004) Biosynthesis of indole-3-acetic acid by the gall-inducing fungus Ustilago esculenta. J Biol Sci 4:744–750
Chutima R, Lumyong S (2012) Production of indole-3-acetic acid by Thai native orchid-associated fungi. Symbiosis 56:35–44
Ditengou FA, Béguiristain T, Lapeyrie F (2000) Root hair elongation is inhibited by hypaphorine, the indole alkaloid from the ectomycorhizal fungus Pisolithus tinctorius, and restored by indole-3-acetic acid. Planta 211:722–728
Ek M, Ljungquist PO, Stenstrom E (1983) Indole-3-acetic acid production by mycorrhizal fungi determined by gas chromatography-mass spectrometry. New Phytol 94:401–407
Ehmann A (1977) The van Urk-Salkowski reagent-a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr 132:267–276
Elumalai S, Prabhakaran M, Raaman N (2011) Indole acetic acid (IAA) levels of cladodes and roots in effect of mycorrhizal and actinorhizal inoculation on C. equisetifolia under glasshouse condition. Recent Res Sci Tech 3:70–75
Felsenstein J (1985) Confidence intervals on phylogenetics: an approach using bootstrap. Evolution 39:783–791
Fomina MA, Alexander IJ, Colpaert JV, Gadd GM (2005) Solubilization of toxic metal minerals and metal tolerance of mycorrhizal fungi. Soil Biol Biochem 37:851–866
Frankenberger WTJ, Poth M (1987) Biosynthesis of indole-3-acetic acid by the pine ectomycorrhizal fungus Pisolithus tinctorius. Appl Environ Microbiol 53:2908–2913
Gadd GM (1993) Interactions of fungi with toxic metals. New Phytol 124:25–60
Gadd GM (2004) Microbial influence on metal mobility and application for bioremediation. Geoderma 122:109–119
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156:609–645
Gay G, Rouillon R, Bernillon J, Favre-Bonvin J (1989) IAA biosynthesis by the ectomycorrhizal fungus Hebeloma hiemale as affected by different precursors. Can J Bot 67:2235–2239
Gharieb MM, Gadd GM (1999) Influence of nitrogen source on the solubilization of natural gypsum (CaSO4.2H2O) and the formation of calcium oxalate by different oxalic and citric acid producing fungi. Mycol Res 103:473–480
Ghosh S, Basu PS (2006) Production and metabolism of indole acetic acid in roots and root nodules of Phaseolus mungo. Microbiol Res 161:362–366
Griffin D (1993) Fungal physiology, 2nd edn. Wiley-Liss, New York
Hasan H (2002) Gibberellin and auxin production by plant root-fungi and their biosynthesis under salinity-calcium interaction. Acta Microbiol Immunol Hung 49:105–118
Jentschke G, Godbold DL (2000) Metal toxicity and ectomycorrhizas. Physiol Plant 109:107–116
Jones MD, Hutchinson TC (1988) Nickel toxicity in mycorrhizal birch seedlings infected with Lactarius rufus or Scleroderma flavidum. I. Effects on growth, photosynthesis, respiration and transpiration. New Phytol 108:451–459
Karabaghli C, Frey-Klett P, Sotta B, Bonnet M, Tacon FL (1998) In vitro effects of Laccaria bicolor S238N and Pseudomonas fluorescens strain BBc6 on rooting of de-rooted shoot hypocotyls of Norway spruce. Tree Physiol 18:103–111
Khamna S, Yokota A, Peberdy JF, Lumyong S (2010) Indole-3-acetic acid production by Streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. EurAsia J BioSci 4:23–32
Kumla J, Suwannarach N, Bussaban B, Lumyong S, Danell E (2012) Basidiome formation of an edible wild, putatively ectomycorrhizal fungus, Phlebopus portentosus without host plant. Mycologia 104:597–603
Lapeyrie F, Ranger J, Vairelles D (1991) Phosphate-solubilizing activity of ectomycorrhizal fungi in vitro. Can J Bot 69:342–346
Machuca A, Pereira G, Aguiar A, Milagres AMF (2007) Metal-chelating compounds produced by ectomycorrhizal fungi collected from pine plantations. Lett Appl Microbiol 44:7–12
Makita N, Hirano Y, Yamanaka T, Yoshimura K, Kosugi Y (2012) Ectomycorrhizal-fungal colonization induces physio-morphological change in Quercus serrata leaves and roots. J Plant Nutr Soil Sci 175:900–906
Mapelli F, Marasco R, Balloi A, Rolli E, Cappitelli F, Daffonchio D, Borin S (2012) Mineral-microbe interactions: biotechnological potential of bioweathering. J Biotechnol 157:473–481
Meharg AA, Cairney JWG (2000) Co-evolution of mycorrhizal symbionts and their hosts to metal-contaminated environments. Adv Ecol Res 30:69–112
Niemi K, Vuorinen T, Ernstsen A, Häggman H (2002) Ectomycorrhizal fungi and exogenous auxins influence root and mycorrhiza formation of Scots pine hypocotyl cuttings in vitro. Tree Physiol 22:1231–1239
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30:1562–1574
Ray P, Tiwari R, Reddy UG, Adholeya A (2005) Detecting the heavy metal tolerance level in ectomycorrhizal in vitro. World J Microbiol Biotechnol 21:309–315
Rudawska M, Kieliszewska-Rokicka B (1997) Mycorrhizal formation by Paxillus involutus in relation to their IAA-synthesizing activity. New Phytol 137:509–517
Splivallo R, Fischer U, Göbel C, Feussner I, Karlovsky P (2009) Truffles regulate plant root morphogenesis via the production of auxin and ethylene1. Plant Physiol 150:2018–2029
Strelczyck E, Pokojska-Burdziej A (1984) Production of auxins and gibberellin-like substances by mycorrhizal fungi, bacteria and actinomycetes isolated from soil and the mycorrhizosphere of pine (Pinus sylvestris L.). Plant Soil 81:185–194
Strelczyck E, Sitek JM, Kowalski S (1977) Synthesis of auxins from tryptophan and tryptophan precursors by fungi isolated from mycorrhizae of pine (Pinus silvestris L.). Acta Microbiol Pol 26:255–264
Swofford DL (2002) PAUP*: Phylogenetic analysis using parsimony (*and 483 other methods), beta version 4.0b10. Sinauer Associates, Sunderland
Tam PCF (1995) Heavy metal tolerance by ectomycorrhizal fungi and metal amelioration by Pisolithus tinctorius. Mycorrhiza 5:181–187
Taylor AFS, Alexander I (2005) The ectomycorrhizal symbiosis: life in the real world. Mycologist 19:102–111
Teale WD, Paponov IA, Palme K (2006) Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7:847–859
Thomson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tomaszewski M, Wojciechowska B (1975) The role of growth regulators released by fungi in pine mycorrhizae. Hirokawa Publishing, Tokyo
Tsavkelova EA, Cherdyntseva TA, Botina SG, Netrusov AI (2007) Bacteria associated with orchid roots and microbial production of auxin. Microbiol Res 162:69–76
Van Tichelen KK, Colpaert JV, Vangronsveld J (2001) Ectomycorrhizal protection of Pinus sylvestris against copper toxicity. New Phytol 150:203–213
Vitorino LC, Silva FG, Soares MA, Souchie EL, Costa AC, Lima WC (2012) Solubilization of calcium and iron phosphate and in vitro production of indoleacetic acid by endophytic isolates of Hyptis marrubioides Epling (Lamiaceae). IRJOB 3:47–54
Vodnik D, Byrne AR, Gogala N (1998) The uptake and transport of lead in some ectomycorrhizal fungi in culture. Mycol Res 102:953–958
Watling R (2006) The sclerodermatoid fungi. Mycoscience 47:18–24
White TJ, Bruns TD, Lee S, Taylaor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Wilson AW, Binder M, Hibbett DS (2011) Effect of gasteroid fruiting body morphology on diversification rate in three independent clades of fungi estimated using binary state speciation and extinction analysis. Evolution 65:1305–1322
Yurekli F, Geckil H, Topcuoglu F (2003) The synthesis of indole-3-acetic acid by the industrially important white-rot fungus Lentinus sajor-caju under different culture conditions. Mycol Res 107:305–309
Zaghian S, Shokri D, Emtiazi G (2012) Co-production of UV-stable bacteriocin-like inhibitory substance (BLIS) and indole-3-acetic acid hormone (IAA) and their optimization by Taguchi design in Bacillus pumilus. Ann Microbiol 62:1189–1197
Zhao ZR, Wu ZL, Huang GQ, Li GR (1992) An improved disk bioassay for determining activities of plant growth regulators. J Plant Growth Regul 11:209–213
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Acknowledgments
This work was supported by grants from The Thailand Research Fund for The Royal Golden Jubilee Ph.D. Program (PHD/0309/2550), Research Team Promotion Grant RTA5580007, Chiang Mai University and the Graduate School of Chiang Mai University. We are grateful for the help of Keegan H. Kennedy, Department of Biology, Chiang Mai University, for improving the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumla, J., Suwannarach, N., Bussaban, B. et al. Indole-3-acetic acid production, solubilization of insoluble metal minerals and metal tolerance of some sclerodermatoid fungi collected from northern Thailand. Ann Microbiol 64, 707–720 (2014). https://doi.org/10.1007/s13213-013-0706-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-013-0706-x