Abstract
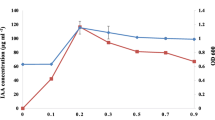
A total of 216 bacterial strains were isolated from rice rhizospheric soils in Northern Thailand. The bacterial strains were initially tested for solubilization of inorganic phosphate, indole acetic acid (IAA) production, selected strains were then tested for optimized conditions for IAA production and whether these caused stimulatory effects on bean and maize seedling growth. It was found that all strains had solubilized inorganic phosphate (P), but only 18.05% produced IAA. The best IAA producer was identified by biochemical testing and 16S rDNA sequence analysis as Klebsiella SN 1.1. In addition to being the best IAA producer, this strain was a high P-solubilizer and produced the highest amount of IAA (291.97 ± 0.19 ppm) in culture media supplemented with l-tryptophan. The maximum production of IAA was achieved after 9 days of incubation. The culture requirements were optimized for maximum IAA production. The tested of IAA production by selected isolates was studied in a medium with 0, 0.1, 0.2, 0.5, 0.7, and 0.9% (v/v) l-tryptophan. Low levels (12.6 ppm) of IAA production was recorded without tryptophan addition. Production of IAA in Klebsiella SN 1.1 increased with an increase to 0.2% (v/v) tryptophan concentration. The production of IAA was further confirmed by extraction of crude IAA from this isolate and subsequent Thin Layer Chromatography (TLC) analysis. A specific spot from the extracted IAA production was found to correspond with a standard spot of IAA with the same R f value. The Klebsiella strain SN 1.1 also demonstrated stimulatory effects on bean seedlings in vivo.



Similar content being viewed by others
Abbreviations
- CFU:
-
Colony forming unit
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- nm:
-
Nanometre
- OD:
-
Optical density
- rev min-1 :
-
Revolution per minute
- rpm:
-
Round per minute
- SD:
-
Standard deviation
- TLC:
-
Thin layer chromatography
- v/v:
-
Volume per volume
- w/v:
-
Weight per volume
References
Ahmad F, Ahmad I, Khan MS (2005) Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of Tryptophan. Turk J Biol 29:29–34
Arora S, Kaur K, Kaur S (2003) Indian medicinal plants as a reservoir of protective phytochemicals. J Environ Pathol Toxicol Oncol 1:295–300
Barea JM, Navorro E, Montoya E (1976) Production of plant growth regulators by rhizosphere phosphate solubilizing bacteria. J Appl Bacteriol 40:129–134
Beyeler M, Michaux P, Keel C, Haas D (1997) Effect of enhanced production of indole-3-acetic acid by the biological control agent Pseudomonas fluorescens CHAO on plant growth. In: Proceedings of the fourth international workshop on plant growth promoting rhizobacteria, Sapporo University, Sapporo, Japan, 18–22 October
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Brown ME (1972) Plant growth substances produced by microorganism of soil and rhizosphere. J Appl Bacteriol 35:443
Chanway CP (2002) Plant growth promotion by Bacillus and relatives. In: Berkeley R, Heyndrickx M, Logan N, De Vos P (eds) B. subtilis for biocontrol in variety of plants. Blackwell Publishing, Boston, MA, pp 219–235
Cisse L, Amao B (2000) The importance of phosphate fertilizer for increased crop production in developing countries. In: AFA 6th international annual conference, Cairo, Egypt, 31 January–2 February 2000
Cleland R (1971) Cell wall extension. Annu Rev Plant Phys 22:197–222
Datta C, Basu PS (2000) Indole acetic acid production by a Rhizobium species from root nodules of a leguminous shrub, Cajanus cajan. Microbiol Res 155:123–127
De Freitas JR, Banerjee MR, Germida JJ (1997) Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). Biol Fertil Soils 24:358–364
Dosselaere F, Vande Broek A, Lambrecht M, De Troch P, Prinsen E, Okon Y, Keijers V, Vanderleyden J (1997) Indole-3-acetic acid biosynthesis in Azospirillum brasilense. In: Proceedings of the fourth international workshop on plant growth-promoting rhizobacteria, Sapporo, Japan
Ehmann A (1977) The van Urk-Salkowski reagent—a sensitive and specific chromogenic reagent for silica gel thin layer chromatographic detection and identification of indole derivatives. J Chromatogr 132:267–276
El-Khawas H, Adachi K (1999) Identification and quantification of auxins in culture media of Azospirillum and Klebsiella and their effect on rice roots. Biol Fertil Soils 28:377–381
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Flaishman MA, Eyal A, Ziberstein C, Voisard C, Hass D (1996) Suppression of Septoria tritici blotch and leaf rust of wheat by recombinant cyanide-producing strains of Pseudomonas putida. Mol Plant Microbe Interact 9:642–645
Frankenberger WT, Poth M (1987) Biosynthesis of indole-3-acetic acid by de pine ectomycorrhizal fungus Pisolithus tinctorius. App Environ Microbiol 53:2908–2913
Gargova S, Roshkova Z, Vancheva G (1997) Screening of fungi for phytase production. Biotechnol Tech 11:221–224
Ghosh S, Basu PS (2006) Production and metabolism of indole acetic acid in roots and root nodules of Phaseolus mungo. Microbiol Res 161:362–366
Glick BR, Karaturovic DM, Newell PC (1995) A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can J Microbiol 41:533–536
Holt JG, Krieng NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams and Wilkins Publishers, Baltimore
Johri JK, Surange S, Nautiyal CS (1999) Occurrence of salt, pH and temperature tolerant phosphate-solubilizing bacteria in alkali soils. Curr Microbiol 39:89–93
Kennedy LR, Pereg-Gerk C, Wood R, Deaker K, Gichrist K, Katupitiya S (1997) Biological nitrogen fixation in non-leguminous field crops: facilitating the evolution of an effective association between Azospirillum and wheat. Plant Soil 194:65–79
Khan MMK, Bhuiyan M, Kabir SM, Oki Y, Adachi T (2006) Effect of selected treatments on the production of rice (Oryza sativa) in acid sulfate soils in a stimulation study. Jpn J Trop Agric 50:109–115
Leinho V, Vacek O (1994) Biosynthesis of auxin by phosphate solubilizing rhizobacteria from wheat (Triticum aestivum) and rye (Secale cereale). Microbiol Res 149:31–35
Mishra R (1968) Ecology workbook. Oxford & IBA, Calcutta
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Pidiyar VJ, Jangid K, Patole MS, Shouche YS (2004) Studies on cultured and uncultured RNA gene analysis. Am J Trop Med Hyg 70:597–603
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rueda-Pente EO, Troyo-Dieguez E, Diaz de Leon-Alvarez JL (2004) Effect of Klebsiella pneumoniae and Azospirillum halopraeferens on the growth and development of two Saliconia bigelovii genotypes. Aust J Exp Agr 44:65–74
Sokal RP, Rohlf FJ (1995) Biometry: the principle and practice of statistics in biological research, 3rd edn. W.H Freeman and Company, New York, pp 1–887
Van Overbeek LS, Van Veen JA, Van Elsas JD (1997) Induced reporter gene activity, enhanced stress resistance, and competitive ability of a genetically modified Pseudomonas fluorescens strain released into a field plot planted with wheat. Appl Environ Microbiol 63:1965–1973
Vijila K (2000) Estimation of IAA production in nitrogen fixing microorganisms. Practical Manual-Microbial interaction in Soil. Tamil Nadu Agricultural University, Coimbatore, pp 38–39
Acknowledgments
Grants for PhD research and Target Research Program (NRU) from The Commission of Higher Education and the Graduate School of Chiang Mai University are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaiharn, M., Lumyong, S. Screening and Optimization of Indole-3-Acetic Acid Production and Phosphate Solubilization from Rhizobacteria Aimed at Improving Plant Growth. Curr Microbiol 62, 173–181 (2011). https://doi.org/10.1007/s00284-010-9674-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9674-6