Abstract
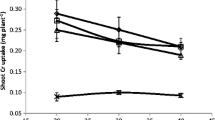
The capacity of nodulating bacteria to survive in soil containing various heavy metal elements has been investigated with the aim of promoting the revegetation of mining sites with Medicago sp. Soil samples were collected from three different mining sites and one agricultural site at a location north of Tunisia. Heavy metal composition analysis showed that the soil samples were contaminated with different concentrations of cadmium (Cd), copper (Cu), lead (Pb) and zinc (Zn). The forage plant Medicago sativa was able to grow normally and to develop effective nodules in these contaminated soils. Sinorhizobium sp. strains nodulating Medicago sativa plants grown in these mining soil samples were isolated and characterized. The isolated strains were able to grow in soils containing up to 2.5 mM Zn, 0.3 mM Cd, 1 mM Cu and 2 mM Pb. The bioaccumulation was tested for two contrasting strains for each metal. For Cd, Pb, and Zn, strain S532 (tolerant strain) adsorbed lower amounts of metals than sensitive strain S112. For Cu, tolerant strain S412 absorbed more Cu than sensitive strain S112, even though adsorption was similar for these two strains. Our results support the use of Medicago sativa–sinorhizobium symbiosis for the regeneration and enrichment of moderately contaminated soils.


Similar content being viewed by others
References
Andronov EE, Roumyantseva ML, Sagoulenko VV, Simarov BV (1999) Effect of the host plant on the genetic diversity of a natural population of Sinorhizobium meliloti. Russ J Genet 35:1169–1176
Badri M, Ilahi H, Huguet T, Aouani ME (2007) Quantitative and molecular genetic variation in sympatric populations of Medicago laciniata and M. truncatula (Fabaceae): relationships with eco-geographical factors. Genet Res Camb 89:107–122
Brimecombe MJ, De Leij FA, Lynch JM (2001) The effect of root exudates on rhizosphere microbial populations. In: Pinto R, Varanini Z, Nannipierei P (eds) The rhizosphere. Marcel Dekker, New York, pp 95–141
Broos K, Uyttebroek M, Martens J, Smolders E (2004) A survey of symbiotic nitrogen fixation by white clover grown on metal contaminated soils. Soil Biol Biochem 36:633–640
Broos K, Beyens H, Smolders E (2005) Survival of rhizobia in soil is sensitive to elevated zinc in the absence of the host plant. Soil Biol Biochem 37:573–579
Brunel B, Rome S, Ziani R, Cleyet-Marel JC (1996) Comparison of nucleotide diversity and symbiotic properties of Rhizobium meliloti populations from annual Medicago species. FEMS Microbiol Ecol 19:71–82
Carrasco JA, Armanio P, Eajuelo P, Burgos A, Caviedes MA, López R, Chamber MA, Palomares AJ (2005) Isolation and characterization of symbiotically effective rhizobium resistant to arsenic and heavy metals after the toxic spill at the Aznalcollar pyrite mine. Soil Biol Biochem 37:1131–1140
Dary M, Chamber-Pérez MA, Palomares AJ, Pajuelo E (2010) “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard Mater 177:323–330
Del Rio M, Font F, Almela C, Vélez D, Motoro R, De Haro-Bailon A (2002) Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcollar mine. J Biotechnol 98:125–137
Delorme S, Philippot L, Edel-Hermann V, Deulvot C, Mougel C, Lemanceau P (2003) Comparative genetic diversity of the narG, nosZ and 16S rRNA genes in fluorescent Pseudomonas. Appl Environ Microbiol 69:1004–1012
Eardly BD, Materon LA, Smith NH, Johnson DA, Rumbaugh MD, Selander RK (1990) Genetic structure of natural populations of the nitrogen-fixing bacterium Rhizobium meliloti. Appl Environ Microbiol 56:187–194
Gadd GM (1992) Metals and microorganisms: a problem of definitions. FEMS Microbiol Lett 100:197–204
Gardea-Torresdey JL, Teimann KJ, Gonzalez JH, Cano-Aquilera I, Henning JA, Townsend MS (1996) Removal of nickel ions from aqueous solutions by biomass and silica immobilized Medicago sativa. J Hazard Mater 49:205–216
Gerhardt KE, Huang XD, Glick BR, Greenberg BM (2009) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176:20–30
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol Biochem 30:1389–1414
Glick BR (2004) Changes in plant growth and development by rhizosphere bacteria that modify plant ethylene levels. Acta Hortic 631:265–273
Ibekwe AM, Angle JS, Chaney RL, van Berkum P (1997) Differentiation of clover Rhizobium isolated from biosolids amended soils with varying pH. Soil Sci Soc Am J 61:1679–1685
Jebara M, Mhamdi R, Aouani ME, Ghrir R, Mars M (2001) Genetic diversity of Sinorhizobium populations recovered from different Medicago varieties cultivated in Tunisian soils. Can J Microbiol 47:139–147
Khan MS, Zaidi A, Wani PA, Ahemad M, Oves M (2009) Functional diversity among plant growth-promoting Rhizobacteria. In: Khan MS, Zaidi A, Musarrat J (eds) Microbial strategies for crop improvement. Springer, Berlin, Heidelberg, pp 105–132
Klavins M, Briede A, Rodinov V, Kokorite I, Parele E, Klavina I (2000) Heavy metals in rivers of Latvia. Sci Total Environ 262:175–183
Lopez ML, Peralta-Videa JR, Benitez T, Gardea-Torresdey JL (2005) Enhancement of lead uptake by alfalfa (Medicago sativa) using EDTA and a plant growth promoter. Chemosphere 61:595–598
McGrath SP, Chaudri AM, Giller KE (1995) Long-term effects of metals in sewage sludge on soils, microorganisms and plants. J Ind Microbiol 14:94–104
Pajuelo E, Rodriguez-Llorente ID, Dary M, Palomares AJ (2008) Toxic effects of arsenic on Sinorhizobium–Medicago sativa symbiotic interaction. Environ Pollut 154:203–211
Pereira SIA, Lima AIG, Figueira EM, de Almeida P (2006) Heavy metal toxicity in Rhizobium leguminosarum biovar viciae isolated from soils subjected to different sources of heavy-metal contamination: Effects on protein expression. Appl Soil Ecol 33:286–293
Robinson RA, Wilson JD, Crick HQP (2001) The importance of arable habitat for rarmland birds in grassland landscapes. J Appl Microbiol 38:1059–1069
Rodríguez-Llorente ID, Dary M, Gamane D, El Hamdaoui A, Doukkali B, Lafuente A, Delgadillo J, Caviedes MA, Pajuelo E (2010) Cadmium biosorption properties of the metal resistant Ochrobactrum cytisi Azn6.2. Eng Life Sci 10:49–56
Rough DA, Lee BTO, Morby AP (1995) Understanding cellular responses to toxic agents: a model for mechanism-choice in bacterial metal resistance. J Ind Microbiol 14:132–141
Saidi S, Zribi K, Badri Y, Aouani ME (2009) Genetic characterization and symbiotic proprieties of native sinorhizobia trapped by Medicago sativa on Tunisian soils. Aust J Soil Res 47:321–327
Stacey G, McAlvin CB, Kim S-Y, Olivares J, Soto MJ (2006) Effects of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago truncatula. Plant Physiol 141:1473–1481
Vincent JM (1970) A manual for practical study of root-nodule bacteria. IBP handbook 15. Blackwell Scientific, Oxford
Zhuang XL, Chen J, Shim H, Bai Z (2007) New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int 33:406–413
Zribi K, Mhamdi R, Huguet T, Aouani ME (2005) Diversity of Sinorhizobium meliloti and S. medicae nodulating Medicago truncatula according to host and soil origins. World J Microbiol Biotechnol 21:1009–1015
Acknowledgements
The authors thank Dr. Issam Nouairi in the laboratory of legumes (CBBC) for fruitful discussion. Our thanks are also addressed to Hedi Hamrouni, INGREF, and Ons Talbi, Laboratory of Extremophile Plants (CBBC), for soil analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zribi, K., Djébali, N., Mrabet, M. et al. Physiological responses to cadmium, copper, lead, and zinc of Sinorhizobium sp. strains nodulating Medicago sativa grown in Tunisian mining soils. Ann Microbiol 62, 1181–1188 (2012). https://doi.org/10.1007/s13213-011-0358-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0358-7