Abstract
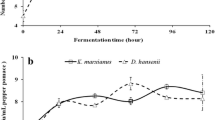
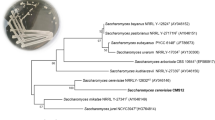
Two myrosinase-producing fungi, Lichtheimia sp. JN3C and Aspergillus terreus, were newly isolated from decayed rapeseed meal samples obtained in Anhui Province, China. After preliminary screening, re-screening and combination of two screened strains with a yeast, an optimal composite strain to ferment rapeseed meal was obtained. Results demonstrated that the glucosinolate content of products with two molds fermentation was overall lower than that with single strain fermentation. Fermentation with composite strains containing Candida tropicalis CICIM Y0079(T) had a similar glucosinolate content, whereas the protein content was remarkably increased compared to two molds fermentation. Under sterile conditions, a 96-h fermentation with the composite strains resulted in the degradation of 66.2% of crude fiber, 28.3% of phytic acid, and 98% of total glucosinolates, which are responsible for goiter, and an increase of the protein and tannins content by 27.4 and 15.8%, respectively. In addition, glucosinolates and protein content under the non-sterile condition were not significantly different compared to the sterile condition. The fermentation greatly improved the nutritional quality of rapeseed meal by both degrading undesired substances and increasing protein content.
Similar content being viewed by others
References
Anupama R, Ravindra P (2000) Value-added food: single cell protein. Biotechnol Adv 18(6):459–479
Barrette J, Gélinas P (2007) Protein enrichment of potato processing waste through yeast fermentation. Bioresour Technol 98:1138–1143
Bau HM, Villaume C, Lin CF, Evrard J, Quemener B, Nicolas JP, Mkjean L (1994) Effect of a solid-state fermentation using Rhizopus oligosporus sp.T-3 on elimination of antinutritional substances and modification of biochemical constituents of defatted rapeseed meal. J Sci Food Agric 65:315–322
Bhat TK, Singh B, Sharma OP (1998) Microbial degradation of tannins – a current perspective. Biodegradation 9:343–357
Butrindr B, Niamsup H, Shank L, Rakariyatham N (2004) Myrosinase overproducing mutants of Aspergillus sp. NR463. Ann Microbiol 54(4):93–501
Das MM, Singhal KK (2005) Effect of feeding chemically treated mustard cake on growth, thyroid and liver function and carcass characteristics in kids. Small Ruminant Res 56:31–38
Dvorakova J (1998) Phytase, sources, preparation and exploitation. Folia Microbiol 43(4):323–338
Ebune A, Al-Asheh S, Duvnjak Z (1995) Production of phytase during solid-state fermentation using Aspergillus Ficuum NRRL 3135 in canola meal. Bioresour Technol 53(1):7–12
Ezekiel OO, Aworh OC, Blaschek HP, Ezeji TC (2010) Protein enrichment of cassava peel by submerged fermentation with Trichoderma viride (ATCC 36316). Afr J Biotechnol 9(2):187–194
Fahey JW, Wade KL, Stephenson KK, Chou FE (2003) Separation and purification of glucosinolates from crude plant homogenates by high-speed counter-current chromatography. J Chromatogr A 996:85–93
Hagerman AE, Butler LG (1978) Protein precipitation method for the quantitative determination of tannins. J Agric Food Chem 26:809–812
Haug W, Lantzsch HJ (1983) Sensitive method for the rapid determination of phytate in cereals. J Agric Food Chem 34:1423–1426
Joseph I, Paulraj R, Bhatnagar D (2008) Effect of solid state fermentation on nutrient composition of selected feed ingredients. Indian J Fish 55(4):327–332
Lateef A, Oloke JK, Kana EBG, Oyeniyi SO, Onifade OR, Oyeleye AO, Oladosu OC, Oyelami AO (2008) Improving the quality of agro-wastes by solid-state fermentation: enhanced antioxidant activities and nutritional qualities. World J Microbiol Biotechnol 24:2369–2374
Mińkowski K (2002) Influence of dehulling of rapeseeds on chemical composition of meal. Anim Feed Sci Technol 96:237–244
Mithen RF, Dekker M, Verkerk R, Rabot S, Johnson IT (2000) The nutritional significance, biosynthesis and bioavailability of glucosinolates in human foods. J Sci Food Agric 80:967–984
Naczk M, Nichols T, Pink D, Sosulski F (1994) Condensed tannins in canola hulls. J Agic Food Chem 42:2196–2200
Oboh G (2006) Nutrient enrichment of Cassava peels using a mixed culture of Saccharomyces cerevisiae and Lactobacillus spp. Solid media fermentation techniques. Electron J Biotechnol 9:46–49
Palop ML, Smiths JP, Brink B (1995) Degradation of sinigrin by Lactobacillus agilis strain R16. Int J Food Microbiol 26:219–229
Rajesh N, Imelda-Joseph RRP (2010) Value addition of vegetable wastes by solid-state fermentation using Aspergillus niger for use in aquafeed industry. Waste Manag 30(11):2223–2227
Rakariyatham N, Sakorn P (2002) Biodegradation of glucosinolates in brown mustard seed meal (Brassica juncea) by Aspergillus sp. NR-4201 in liquid and solid-state cultures. Biodegradation 13:395–399
Rakariyatham N, Butr-indr B, Niamsup H, Shank L (2005) Screening of filamentous fungi for production of myrosinase. Braz J Microbiol 36:242–245
Rakariyatham N, Butr-Indr B, Niamsup H, Shank L (2006) Improvement of myrosinase activity of Aspergillus sp. NR4617 by chemical mutagenesis. Electron J Biotechnol 9(4):379–385
Rozan P, Villaume C, Bau HM, Schwertz A, Nicolas JP, Méjean L (1996) Detoxication of rapeseed meal by Rhizopus oligosporus sp-T3: A first step towards rapeseed protein concentrate. Int J Food Sci Technol 31:85–90
Sakorn P, Rakariyatham N, Niamsup H, Kovitaya P (1999) Sinigrin degradation by Aspergillus sp. NR-4201 in liquid culture. Sci Asia 25:189–194
Sakorn P, Rakariyatham N, Niamsup H, Nongkunsarn P (2002) Rapid detection of myrosinase-production fungi: a plate method based on opaque barium sulphate formation. World J Microbiol Biotechnol 18:73–74
Smits JP, Knol W, Bol J (1993) Glucosinolate degradation by Aspergillus clavatus and Fusarium oxysporum in liquid and solid-state fermentation. Appl Microbiol Biotechnol 38:696–701
Travers-Martin N, Kuhlmann F, Müller C (2008) Determination of free and complexed myrosinase activities in plant extracts. Plant Physiol Biochem 46:506–516
Vig AP, Walia A (2001) Beneficial effects of Rhizopus oligosporus fermentation on reduction of glucosinolates, fiber and phytic in rapeseed (Brassica napus) meal. Bioresour Technol 78:309–312
Wallig MA, Belyea RL, Tumbleson ME (2002) Effect of pelleting on glucosinolates content of Crambe meal. Anim Feed Sci Technol 99:205–214
Wathelet J-P, Wagstaffe PJ, Biston R, Marlier M, Severin M (1988) Rapeseed reference materials for glucosinolate analysis. Fresen J Anal Chem 332:689–693
Wilkinson AP, Rhodges MJC, Fenwick GR (1984) Determination of myrosinase (thioglucoside glucohydrolase) activity by spectrophotometric coupled enzyme assay. Anal Biochem 139:284–291
Wodzinski RJ, Ullah AHJ (1996) Phytase. Adv Appl Microbiol 42:263–302
Wu B, Zhang G, Shuang S, Dong C, Choi MMF, Lee AWM (2004) A biosensor with myrosinase and glucose oxidase bienzyme system for determination of glucosinolates in seeds of commonly consumed vegetables. Sens Actuat B 106:700–707
Xiao L, Yang LY, Zhang Y, Gu YF, Jiang LJ, Qin BQ (2009) Solid state fermentation of aquatic macrophytes for crude protein extraction. Ecol Eng 35:1668–1676
Acknowledgement
The work is supported by the National Key Technology R&D Program in the 11th Five year Plan of China (Contract No: 2009BADB9B08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Jin, Q., Wang, T. et al. Screening of glucosinolate-degrading strains and its application in improving the quality of rapeseed meal. Ann Microbiol 62, 1013–1020 (2012). https://doi.org/10.1007/s13213-011-0341-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0341-3