Abstract
Innumerable studies associated with cellular differentiation, tissue response and disease modeling have been conducted in two-dimensional (2D) culture systems or animal models. This has been invaluable in deciphering the normal and disease states in cell biology; the key shortcomings of it being suitability for translational or clinical correlations. The past decade has seen several major advances in organoid culture technologies and this has enhanced our understanding of mimicking organ reconstruction. The term organoid has generally been used to describe cellular aggregates derived from primary tissues or stem cells that can self-organize into organotypic structures. Organoids mimic the cellular microenvironment of tissues better than 2D cell culture systems and represent the tissue physiology. Human organoids of brain, thyroid, gastrointestinal, lung, cardiac, liver, pancreatic and kidney have been established from various diseases, healthy tissues and from pluripotent stem cells (PSCs). Advances in patient-derived organoid culture further provides a unique perspective from which treatment modalities can be personalized. In this review article, we have discussed the current strategies for establishing various types of organoids of ectodermal, endodermal and mesodermal origin. We have also discussed their applications in modeling human health and diseases (such as cancer, genetic, neurodegenerative and infectious diseases), applications in regenerative medicine and evolutionary studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell culture has been among the essential articulations used to study the mechanisms by which cells assemble into tissues and organs. Although two-dimensional (2D) cell culture is one of the major techniques through which researchers have elucidated a variety of aspects of cellular interactions, the uniform distribution of cells in the petri dish and the absence of nutrient gradients tend to diminish its physiological relevance (Bonnier et al. 2015). Thus, these conditions may not accurately recapitulate the in vivo architecture of cells and tissues (Bonnier et al. 2015). Increasing the dimensionality of the extracellular matrix (ECM) in a three-dimensional context will significantly impact cell survival, differentiation and proliferation.
Three-dimensional (3D) cell culture involves an artificially created growth environment where cells can interact in all three dimensions. 3D cell culture systems have special importance in fields such as drug discovery, as 2D culture models may not accurately reflect the response of a tissue to a particular compound (Joseph et al. 2019). Increasing evidence suggests that many promising drugs which had passed through the 2D monolayer screening process might have failed as the cellular microenvironment can alter response to the drugs (Hutchinson and Kirk 2011; Edmondson et al. 2014). Furthermore, tissues consist of multiple types of cells of diverse origin organized spatially and temporally along with the extracellular matrix (ECM); whereas many 2D culture systems generally involve multiple cell types with ECM often laid down by the cells itself or provided externally. As such, 3D cell culture models are more representative of in vivo tissue biology and may serve as a physiological model to study human diseases. One such 3D cell culture technique is organoid culture.
Organoids may be defined as cellular aggregates derived from primary tissues or stem cells that can self-organize into organotypic structures. Characteristically organoids comprise more than one cell type of the organ it represents; showcases physiological functions that are specific to that organ; and the cell organization similar to that of the organ itself (Stevens et al. 2009a). Frequently, organoids are grown in complex natural and artificial scaffolds, may show normal tissue architecture, and are highly heterogeneous. Therefore, each organoid generated is unique and exhibits random relative tissue positioning, possibly due to a lack of an embryonal axis for the cells to receive positional cues or signals (Lancaster et al. 2013). The heterogeneity in organoid cultures makes it difficult to obtain single-cell type cultures but has the potential to be an important method in drug development and diseases modeling at the organ level (Eiraku et al. 2011; Kondo and Inoue 2019).
Figure 1 outlines the applications of organoids in basic and translational research. For prospective drug development programs, cell-based assays are essential to assess the potential efficacy (Edmondson et al. 2014). 3D cell culture has been demonstrated to recapitulate in vivo tissue response more accurately than 2D culture. For example, tumoroids, which are 3D cancer models developed in vitro, demonstrate increased resistance to anticancer drugs (Loessner et al. 2010). This increased resistance of 3D models to treatment could be attributed to cell-ECM interaction, decreased drug penetration, and hypoxia, which are known to activate key genes promoting cell survival (Trédan et al. 2007). Further, organ-on-chip technology, which incorporates microfluidic systems into organoid culture, mimics vascularization and further recapitulating in vivo tissue architecture (Van Den Berg et al. 2019).
With advances in cellular reprogramming and personalized medicine strategies, one of the most exciting applications of organoids in disease modeling is biobanking. Organoid biobanks are collections of genetically and histologically characterized organoid models of disease states with matched controls collected from many individuals and this may contribute to advances in both basic and personalized medicine research. These biobanks have been especially valuable in modeling cancer (Schutgens and Clevers 2020). While this can be extended to a variety of disorders and conditions; however, till date, only one non-cancer organoid biobanks is reported which is the repository of intestinal organoid derived from cystic fibrosis subjects (Schutgens and Clevers 2020).
Thus, organoid cultures provide a robust platform through which numerous diseases can be studied; is a better physiological disease model in drug testing than 2D cell culture system and could also bridge the gap in drug response between cellular models and clinical trials (Kondo and Inoue 2019). Various advantages and disadvantages of 3D culture systems have been listed in Table 1.
Current organoid culture strategies
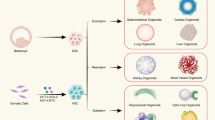
Organoids can be developed from pluripotent stem cells (PSCs), which can be embryonic (ESCs), induced (iPSCs), and adult stem cells (ASCs). Knowledge generated through the innumerable studies performed in the field of developmental biology have paved way for the establishment of protocols to culture various differentiated cell types from stem cell progenitors. Figure 2 indicates some of the key organoid models generated from PSCs and the principal growth factors involved in their generation.
Brief overview of the various organoids generated along with the principal growth factors involved in the development of these organoids from pluripotent stem cells (Modified form Li et al. 2020a)
Ectoderm
Brain organoids
The central nervous system (CNS) of vertebrates is derived from the ectoderm after inductive signaling from the mesoderm. Su et al. (2006) pioneered the use of SFEBq (serum-free floating culture of embryoid bodies with quick reaggregation) in combination with bone morphogenic protein (BMP) and Wnt3a to induce embryonic stem cells (ESCs) to adopt a cerebellar fate. Eiraku and Sasai (2012) designed a SFEBq by placing the reaggregates in a minimal or serum-free media for a week, and then plating again in adhesion plates to promote self-aggregation of cells into spheroids. The ESCs then differentiate into a continuous neuroectoderm-like epithelium. This structure eventually forms stratified cortical tissues. These stratified tissues generally contain cortical progenitors, superficial-layered and deep-layered cortical neurons. When grown in media devoid of growth factors, the cells will spontaneously develop a hypothalamic fate. Other studies have shown that different regions of the brain can be generated by mimicking the endogenous growth factor patterning in vitro. Hedgehog signaling has been shown to drive ventralization of telencephalic progenitors/organoids developed from differentiation from ESCs that later form forebrain (Danjo et al. 2011). Lancaster et al. (2013) developed a method to generate “mini brains” or cerebral organoids containing representations of various regions of the brain. The method begins with embryoid bodies, and growth factors are not used to drive the differentiation of specific tissue lineages. The cells were embedded in Matrigel to allow for the growth of neuroepithelial buds, which in turn spontaneously give rise to various regions of the brain (Lancaster et al. 2013). Cakir et al. (2019) established a method to generate cortical organoids containing a vascular-like network by engineering hESCs to ectopically express ETS variant 2 (ETV2). These vascularized organoids acquired several blood–brain barrier characteristics and supported the formation of perfused blood vessels in vivo (Cakir et al. 2019). Pasca and co-workers generated 3D model in the absence of Matrigel which they called cortical spheroids. They developed brain organoids from embryoid bodies generated by PSCs maintained in non-adherent wells with high-dose ROCK inhibitor for 24 h. This was followed by neural induction using dorsomorphin and SB-431542 up to 5 days. Spheroids generated on the sixth day were then grown on neural media with FGF2 and EGF. These were differentiated into brain organoids comprising astrocytes and cortical neurons by the addition of brain-derived neurotropic factor (BDNF) and neurotropin 3 (NT3) (Pasca et al. 2015).
The human brain is sub-divided into three distinct regions: forebrain, midbrain and hindbrain. Forebrain, the largest of the three regions, comprises of epithalmus, hypothalamus, subthalamus, thalamus and cerebrum. Cerebral aqueduct, cerebral peduncle, tectum and tegmentum form the midbrain whereas the hindbrain comprises cerebellum, medulla oblongata and pons.
Forebrain organoids
Paşca and colleagues have also generated region-specific organoids that resembled the dorsal and ventral forebrain, called cortical and subpallial spheroids respectively, which showed a transition from fetal to postnatal phenotype at around the 280th day in vitro (Trevino et al. 2020). The method developed by Pasca and his team to generate forebrain organoids was built upon from the previously described protocols which involved the use of SMAD pathway inhibitors to induce neural differentiation in hPSCs followed by addition of ROCK inhibitors to aggregate the cells (Birey et al. 2017; Sloan et al. 2018). Briefly, hPSC colonies were lifted using enzyme dispase and cultured in non-adherent plates as suspension cultures. For the generation of dorsal forebrain spheroids, SMAD inhibitors, dorsomorphin (DM) and SB-4321542 (SB) were used for the initial 6 days; followed by addition of EGF and FGF2 growth factors up to 18 days. For maturation, BDNF and NT3 were added for the next 18 days. Ventral forebrain spheroids were generated in a similar fashion from hPSC colonies, altering the specific patterning cues toward ventral fate by adding WNT inhibitor (IWP-2), smoothened agonist (SAG), retinoic acid and allopregnanolone (Birey et al. 2017; Sloan et al. 2018).
Camp et al. (2015) discovered that cortical cells of cerebral organoids showcased gene expression profiles that very closely resembled to those of corresponding fetal tissue, indicating that organoids may recapitulate even gene expression patterns of their corresponding in vivo fetal tissue. Methods have also been developed to culture myelinated and unmyelinated neurons, astrocytes and oligodendrocytes of the cortex (Paşca et al. 2015; Marton et al. 2019).
Thalamic organoids
Thalamus helps in processing and relay of sensory information except for smell and mediating them to cortex. This organ is often associated with disorders and diseases related to movement, ataxia, visual loss, etc. Thalamic organoids were generated from the hESCs or iPSCs by differentiating to neuronal lineage using neural induction media containing SB431542 (Activin-like receptor kinase inhibitor), LDN193189 (BMP type I receptor inhibitor) and insulin for 8 days followed by thalamic patterning media containing BMP7 and PD325901 (MEK inhibitor) for another 8 days and subsequently grown in neural differentiation media (Xiang et al. 2020). In this method, thalamic organoids show representative markers such as TCF7L2 (Transcription Factor 7 Like 2), DBX1 (Developing Brain Homeobox Protein 1) and GBX2 (Gastrulation Brain Homeobox 2). Xiang et al. (2019) also showed that thalamus-like brain organoids in combination with cortical organoids can generate reciprocal projections between the two organoids.
Retinal organoids
The retina is a tissue that originates from the neuroectoderm and is the light receptive tissue of the eye. The optic vesicle originates from the diencephalon. The factors important for retinal differentiation include Paired Box 6 (PAX6), FGF1, FGF2, FGF9, PAX2 and Lim homeobox 2 (LHX2) (Heavner and Pevny 2012). Eiraku et al. (2011) grew murine ESCs in a serum-free medium to generate neuroectodermal cells. Matrigel was used to promote the generation of rigid neuroepithelial tissue. The neuroepithelial buds formed from this culture resembled primordial retinal tissue. These were then cut away and cultured in a medium that supports retinal development (Eiraku et al. 2011). The development of the organoid closely mimicked the processes that occur in the formation of the optic cup in vivo, showing proper apical-basal polarity and expressing various retinal and epithelial markers in a spatially correct manner. The organoids were large, and showed morphological features seen in human optic cups such as apical nuclear positioning and a higher proportion of rods and cones. Nakano et al. (2012) observed that the formation of photoreceptors in the organoids could be accelerated by Notch signaling. Völkner et al. (2016) then generated a protocol to generate 3D retinal organoids that do not require the evagination of optic vesicle-like structure, which until then had greatly limited the yield of optic organoids in culture. Chichagova et al. (2019) established a protocol for the development of retinal organoids from hPSCs. These organoids contained all the major retinal cell types and were responsive to light. The study involved the differentiation of hPSCs into retinal organoids via insulin-like growth factor-1 (IGF-1), retinoic acid and triiodothyronine signaling (Chichagova et al. 2019).
Hippocampus organoids
Located in the temporal lobe, the hippocampus has a major role in the learning and memory. Yu and colleagues developed a protocol for the generation of neuronal progenitor cells (NPCs) with hippocampal identity. Embryoid bodies were treated with antagonists of sonic hedgehog pathway and various factors that mimic the patterning of the forebrain, such as transforming growth factor- β (TGF-β) inhibitor SB431542, Dickkopf WNT signaling pathway inhibitor 1 (DKK-1) and Noggin (NOG). Hippocampal dentate gyrus-like granular neurons expressing PROX1 can be produced by treating the NPCs with high concentrations of WNT3a (Yu et al. 2014). Subsequently, hippocampal neurons were also generated by Sakaguchi et al. (2015). Using serum SFEBq as the starting material, they found that culturing cells with BMP4 and WNT agonist CHIR 99,021 induced formation of the choroid plexus. Changing the time window of exposure to these factors was found to induce self-organization of the tissue to form structures resembling the medial pallium. Formation of granule and pyramidal neurons was seen following long-term dissociation culture, both of which are found in the hippocampal region of the brain (Sakaguchi et al. 2015).
Midbrain organoids
Midbrain organoids are generated from embryoid bodies using hydrogels and orbital shakers embedding by sequential patterning or by simultaneous patterning method (Smits and Schwamborn 2020). In the sequential patterning, dual SMAD inhibitors (dorsomorphin, SB431542, LDN193189, A83, NOG) were used along with WNT modulation (CHIR99021) for the midbrain specification step, followed by midbrain floor plate induction using SHH modulation (smoothened agonist, SHH, purmorphamine) and FGF8, and finally, differentiation and maturation using neurotropic factors BDNF and brain-derived neurotropic factor (GDNF). In simultaneous patterning protocol, the midbrain specification and floor plate induction steps were combined together, followed by maturation step similar to sequential method but involving additional supplements (FGF20, TGFβ3, trichostatin A) (Smits and Schwamborn 2020; Galet et al. 2020).
Hindbrain organoids
Cerebellar development is controlled by the isthmic organizer, which is situated in the midbrain-hindbrain boundary. Muguruma et al. (2010) focused on the generation of purkinje cells, which are derived from the neuronal cells of the cerebellar ventricular zone. Using SFEBq, they combined insulin and fibroblast growth factor 2 (FGF2) to cause differentiation of the isthmic organizer, which secretes inductive factors leading to differentiation of cerebellar precursors (Muguruma et al. 2010). In another study by the same lab, it was reported that culturing human ESCs (hESCs) in the presence of FGF19 and stromal cell-derived factor-1 (SDF1) leads to the generation of structures that closely resemble the first trimester cerebellum (Muguruma et al. 2015).
Assembloids
Pasca (2019) described protocols for the generation of brain assembloids which involves mixing of various types of brain organoids or/and cluster of cells that later on synchronizes and organizes spatially (Pasca 2019; Vogt 2021). Three types of brain assembloids were a) multilineage assembloids b) multiregion assembloids c) polarized assembloids (Pasca 2019). Multilineage assembloids uses a combination of organoids of different cell lineages, such as a combination of neuronal cell lineage based spheroids with glial spheroids which may also have neuroimmune cells (microglia) and vasculature (pericytes and endothelial cells). Pasca and his team generated forebrain assembloids, (a multiregion assembloids) by growing together individual dorsal and ventral forebrain spheroids which eventually fuse leading to ventral forebrain spheroid cortical GABAergic neurons integrating with cortical glutamatergic neurons (Sloan et al. 2018; Pasca 2019). Recently, Cederquist et al. (2019) developed method to generate polarized assembloids that showed spatial topographic organization with latero-medial ganglionic eminences, hypothalamus, thalamus and dorsal forebrain regions. This was achieved by mixing together the forebrain organoids with Sonic Hedgehog (SHH) secreting cells. The Sonic Hedgehog gradient enables formation of positional axis in these polarized organoids (Cederquist et al. 2019; Miura and Pașca 2019).
Endoderm
Stomach organoids
The stomach is a tissue that develops from the endoderm. Specifically, it develops from the posterior foregut of the gastrointestinal tract (GIT). McCracken et al. (2014) showed that temporal manipulation of FGF4, WNT3a, epidermal growth factor (EGF), BMP and retinoic acid pathways during 3D culture of hPSCs could be used to generate gastric organoids. They generated endodermal progenitor cells by treating hPSCs with activin. These cells were then differentiated into the foregut fate through addition of WNT3a, FGF4 activators and BMP inhibitors to the culture media. Addition of retinoic acid caused the organoids to move toward a posterior foregut identity. These tissues were converted into gastric organoids by the application of a high concentration of EGF and showcased molecular and morphogenetic development that mimicked the antrum of murine stomach. The organoids thus generated contained mucous cells, gastric glands and pit-like regions, Lgr5+ stem cells and many other endocrine cells of gastric origin. Stomach organoids can also be generated from multipotent adult stem cells (Watson et al. 2014). In the mouse stomach, Lgr5+ stem cells are located at the base of pyloric glands. Culture of these Lgr5+ stem cells generated long-term organoids that closely resembled the mature pyloric epithelium. Another marker used to identify stem cells in the stomach is Tumor necrosis factor receptor superfamily member 19 (TROY). TROY+ cells of human origin can be cultured in vitro to generate organoids resembling tissue from the corpus of the stomach (Stange et al. 2013). Broda et al. (2019) generated a protocol for generation of human antral and fundic gastric organoids from PSCs.
Small intestinal organoids
hPSCs can be differentiated into definitive endodermal cells upon treatment with activin A with 80% efficiency (D’Amour et al. 2005). FGF4 and WNT3a can then be used to promote differentiation of the endodermal cells toward mid/hindgut fates (Spence et al. 2011). The mid/hindgut spheroids, grown initially in a 2D culture system, were then cultured in Matrigel in the presence of EGF, R-spondin (RSPO1) and NOG. Over the next 1–3 months, the organoids expanded and grew to form polarized intestinal epithelium, containing structures that resembled villi and crypt-like regions of proliferation. The epithelia were surrounded by non-polarized caudal type homeobox 2 (CDX2)+ intestinal mesenchyme (Spence et al. 2011). Furthermore, transplantation of intestinal organoids generated from hESCs into mice resulted in the expansion and maturation of the epithelium and mesenchyme, which can be seen by the various differentiated intestinal lineages that were formed from the transplanted organoids (Barker et al. 2010). Recently, Kasendra et al. (2018) developed a method to generate primary human small intestine-on-a-chip from healthy regions of intestinal biopsies.
Lung organoids
The lung as an organ develops from the endodermal germ layer. Specifically, the lung, along with the thyroid, originate from the NKX2-1+ progenitor cells of the ventral foregut. NKX2-1 is a transcription factor that is vital for lung and thyroid development (Minoo et al. 1999). Longmire et al. (2012) established pure populations of Nkx2-1 progenitors that exhibit the differentiation capability of Nkx+ lung/thyroid tissue from mouse ESCs. Jain et al. (2015) established a protocol to culture cells of the distal airway from adult stem cells of Pdgfra-H2B:GFP mice. The alveolar organoids thus generated consisted of type I (gas exchanging) cells and type II (surfactant secreting) cells, but the culture conditions for these cells have not been completely defined, as co-culture with mouse lung fibroblasts is required for growth (Jain et al. 2015). Dye et al. (2015) devised a method to generate lung organoids from hPSCs. The hPSCs were directed into a definitive endodermal fate by Activin-A treatment. After differentiation into definitive endoderm, cells were incubated in foregut media with noggin, SB-431542 (inhibitor of the activin receptor-like kinase receptors), FGF4 and CHIR 99021 (WNT agonist). Spheroids were observed in the culture after 4 days and were transferred to Matrigel to facilitate 3D growth of cells. Simultaneous activation of hedgehog pathway specified the cells toward a lung fate. Mature lung organoids arose upon prolonged exposure to FGF10 in the Matrigel (Dye et al. 2015). Miller et al. (2019) developed a new protocol for the generation of lung organoids from hPSCs. The structures generated from their protocol resembled developing bronchi/bronchioles surrounded by lung mesenchyme, with cells that were expressing alveolar surface markers (Miller et al. 2019).
Thyroid organoids
The thyroid is an organ that develops from the same progenitor cells as the lungs during embryonic development (Minoo et al. 1999). Initial report suggested that transient overexpression of Pax8 and Nkx2-1 were enough to generate thyroid organoids from mouse ESCs (Antonica et al. 2012). The protocol established by Longmire et al. (2012) also generated thyroid organoids upon sequential exposure of progenitors to BMP/FGF4, followed by plating the cells in 3D architecture in Matrigel containing thyroid stimulating hormone (TSH) to induce development of thyroid organoids (Longmire et al. 2012). Building upon this protocol, Kurmann et al. (2015) established a protocol for generating thyroid organoids using mouse PSCs. The thyroid organoids thus produced and upon transplantation into hypothyroid mice, secreted thyroid hormones and rescued the mice with disease phenotype. Human thyroid organoids could be grown from iPSCs using the same protocol (Kurmann et al. 2015). Saito et al. (2018) reported a protocol for the long-term culture of thyroid organoids that was capable of iodide uptake and thyroglobulin synthesis. Transplantation of these organoids into mouse model of hypothyroidism resulted normal thyroid-like tissues (Saito et al. 2018).
Liver organoids
The liver is an organ whose progenitors arise from the foregut endoderm. It is perhaps among the most extensively researched tissues to create organoids due to several key applications (Prior et al. 2019). Human PSCs can be induced to form hepatic endodermal cells by treatment with activin-A, followed by bFGF/BMP4 treatment, in 2D culture. The hepatic cells thus derived from hPSCs were found along with endothelial cells and mesenchymal stem cells. When plated on Matrigel, spontaneous formation of 3D structures was observed. The liver organoids thus generated were vascularized and when transplanted into mice, the blood vessels in the organoids merged with the host vasculature within 48 h. Mice from lethal drug-induced liver toxicity were rescued by transplantation of liver buds generated in vitro (Takebe et al. 2013). Hepatic organoids could also be generated from Lgr5+ stem cells. The organoids could be dissociated and transferred to fresh matrix every 7–10 days for 6 months (Huch et al. 2015). Numerous tissue engineering methods have also been incorporated in the process of liver organoids production (Underhill and Khetani 2018). 3D-bioprinting strategies have been employed to generate liver organoids, with these showing higher liver function than monolayer counterparts (Ma et al. 2016; Norona et al. 2016). Pettinato et al. (2019) co-cultured human adipose microvascular endothelial cells with hiPSCs to produce liver organoids with greater differentiation yield and improved hepatic function across a wide range of parameters. Wang et al. (2019b) published a serum and feeder-free protocol for the establishment of hepatic organoids from ESCs. These cells did not produce teratomas when transplanted into fat pads of immune-deficient mice, enabled repopulation of the liver, and could be used to model alcoholic liver injury (Wang et al. 2019b).
Pancreatic organoids
As with the liver, pancreatic cells showcase an extremely low turnover rate and do not express Lgr5 under physiological conditions. However, pancreatic injury causes robust activation of the WNT signaling pathway and induces Lgr5 expression in the regenerating pancreatic ducts. Pancreatic progenitor organoids could be cultured from single pancreatic ductal cells, under modified mini-gut conditions. These organoids were able to expand fivefold weekly for over 40 weeks. These pancreatic progenitor cells were able to differentiate into ductal cells as well as endocrine cells upon transplantation (Huch et al. 2013). Ware et al. (2016) developed a protocol for the culture of pancreatic organoids using the hanging drop technique. Loomans et al. (2018) designed a protocol to expand adult pancreatic tissue into organoids containing pancreatic progenitor cells, with the potential to differentiate into endocrine tissue. The protocol for the generation of pancreatic organoids included pancreatic normal media that consisted of Noggin, WNT CM, FGF10, EGF, and R-spondin 3, prostaglandin E2 (PGE2) while the tumor media lacked EGF (Driehuis et al. 2020).
Mesoderm
Cardiac organoids
Stevens et al. (2009a, b) developed a technique to generate cardiac patches of size 300–600 µm thick from cardiac cells obtained from differentiating hESCs. Briefly, human ESCs were differentiated into cardiomyocytes in Activin A and BMP-4 rich medium. These cardiomyocytes (CMs) were suspended on a rotary shaker. These patches comprised majorly cardiac cells (up to 80%) and primary epithelium. These aggregates of cells showed electromechanical coupling, but the patches did not survive post-transplantation (Stevens et al. 2009b). One of the reasons for short life of spheroids could be due to the fact that they did not match the heart tissue composition. Human heart contains about 70% of non-cardiomyocytes which are mainly cardiac endothelial cells for the supply oxygen as well as fatty acids to CM and cardiac fibroblasts for structure and maturation of CMs. To overcome the limitations, the cardiac patches comprising cardiomyocytes, endothelial cells and fibroblasts were generated. This second generation 3D patches showed greater stability, maturity and survival over two years after transplantation (Stevens et al. 2009a). Hudson et al. (2012) also generated primitive cardiac cells from hESCs, using a temporal differentiation protocol. Briefly, primitive streak was induced using BMP-4 and Activin-A, followed by inhibition of WNT signaling using small molecule inhibitor IWP-4 to induce cardiogenesis (Hudson et al. 2012). Ravenscroft et al. (2016) and Giacomelli et al. (2020) have found cardiac tri-cellular system better recapitulates the tissue physiology in vivo showing contractile maturity and enhanced inotropic response, with mature electrophysiology, mitochondrial respiration and sarcomere structures. In this protocol, hESC-derived CMs were used along with human primary cardiac microvascular endothelial cells (hCMECs) and human primary cardiac fibroblasts (hCFs) for the generation of cardiac tissue organoids and grown in non-adherent round bottom 96-well plates. Giacomelli et al. (2017) established protocol for cardiac organoids from pluripotent stem cells which followed simultaneous differentiation of CMs and endothelial cells after cardiac mesoderm induction. Pointon et al. (2017) used a high throughput imaging technique to study the effect of a large panel of inotropic compounds on these cardiac organoids with 80% sensitivity and 91% specificity.
Hinson et al. (2015) engineered cardiac tissues from dilated cardiomyopathy (DCM) patients to evaluate role of titin mutants in DCM that cause premature death by heart failure. Stable cardiac tissues were generated from hiPSCs of DCM patients and using RNAseq analyses. Hinson et al. (2015) identified pathogenicity of titin mutants. Richards et al. (2016) showed advanced strategies in developing biophysical microenvironment. In this protocol, investigators used silicon nanowires on hiPSC-derived cardiac spheroids and found increase in contractility, cell junction formation and reduction in spontaneous beat rate of the nanowired spheroids. This may be beneficial in reducing arrhythmic risk post-transplantation (Richards et al. 2016). Richards et al. (2017) developed a scaffold-free method to generate cardiac organoids using human iPSC-derived cardiomyocytes, human umbilical vein endothelial cells (HUVECs) and human cardiac ventricular fibroblasts. Cells were mixed relative to developing and adult heart. The developing heart ratio showed greater contraction amplitude and sarcomere formation (Richards et al. 2017). Hoang et al. (2018) generated cardiac organoids using 3D cardiac microchambers from 2D human iPSC colonies. In another study, Richards et al. (2020) developed a cardiac organ disease model for myocardial infarction (MI). In this model, cardiac microtissues fabricated in agarose hydrogel were placed in hypoxia chamber with 10% O2 and 1 µM noradrenaline up to 10 days to generate infarct organoids (Richards et al. 2020).
Tiburcy et al. (2017) developed an engineered human myocardium (EHM) for disease modeling such as heart failure and repair from ESCs and iPSCs under serum-free conditions. EHM was treated with norepinephrine hydrochloride and endothelin-1 along with ascorbic acid-2-phosphate sesquimagnesuim salt for 7 days to generate heart failure model (Tiburcy et al. 2017). Early maturation of iPSCs derived cardiac tissue can be conditioned to achieve adult human myocardium (Ronaldson-Bouchard et al. 2018, 2019a). In this protocol, the early stage hiPSCs cardiomyocytes (maturation at day 12 from spontaneous contractions) subjected to electromechanical conditioning and with increased induced contractions, resulting in the generation of cardiac tissue that closely recapitulated the adult human myocardium (Ronaldson-Bouchard et al. 2018, 2019a). In another protocol, the engineered cardiac tissue was subjected to increased electromechanical stimulation up to 21 days generating adult like phenotype (Ronaldson-Bouchard et al. 2019b).
Kidney organoids
The kidney originates from the embryonic intermediate mesoderm, which generates two progenitor cell populations, namely, the metanephric mesenchyme and the ureteric epithelium. These two cell populations form the nephrons and collecting ducts by interacting with one another. Initially, both these cell types could not be generated simultaneously, until Takasato and colleagues established a protocol to produce both the principle lineages of the kidney. Activin A and BMP4 were utilized to generate primitive streak identity in 2D culture human PSCs. FGF9 is then added to generate intermediate mesoderm identity. The cells then spontaneously developed into ureteric epithelium and metanephric mesenchyme and showed 3D structures when co-cultured with mouse kidney reaggregates or when cultured at extremely low densities in 2D. In a follow-up study, hPSCs were cultured in 2D with WNT signals for 4 days, after which they were treated with FGF9 for 3 days. These were cultured as organoids for up to 3 weeks. Brief exposure to WNT agonists greatly increased the number of nephrons seen in the organoid culture and results in the formation of kidney organoids with fully segmented nephrons, which were surrounded by renal endothelia and interstitium (Takasato et al. 2014, 2016). Morizane and Bonventre (2017) established a protocol for the generation of nephron progenitor cells with very high efficiency (80–90%) within 9 days of hPSC differentiation. More recently, Homan et al. (2019) developed protocols to culture kidney organoids in 3D-printed millifluidic chips. Their study showed that exposure of organoids to fluid shear stress (FSS) resulted in an enhanced peripheral expression of vascular markers melanoma cell adhesion molecule (MCAM) and platelet endothelial cell adhesion molecule (PECAM). They also showed a tenfold increase in the junctional density (number of branching points per unit area) and average vessel length (interjunctional distance) (Homan et al. 2019).
Application of organoids in human health and disease
The rapid improvement in the methods for culture of patient-derived organoids has permitted disease modeling with greater precision suggesting their potential in translational medicine and personalized therapies. While methods for the development of organoids from PSCs had been established in the beginning of the twenty-first century, ASCs were thought to have limited replicative potential in vitro. One of the key advantages of using organoids to model diseases is that with the advent of iPSC-derived organoids, it is possible to personalize treatment based on the genome of individual patients. The generation of organoids from adult tissues require no genetic transduction, supporting the claim that organoids are a viable avenue for tissue transplantation (Laurent et al. 2011). The molecular identity and histology of organoids can be studied by a variety of techniques such as single-cell RNA sequencing and high-resolution microscopy techniques, such as electron microscopy and confocal microscopy (Stange et al. 2013; Freedman et al. 2015; Matano et al. 2015; Roerink et al. 2018). This allows for a much deeper understanding of stem cell biology, homeostasis in tissues and the pathophysiology of hereditary diseases such as cancer, cystic fibrosis, neurodegenerative and neuropsychiatric disorders and various bacterial and viral diseases (Bartfeld and Clevers 2015; Wells et al. 2016; Wang et al. 2020). Table 2 lists out the various diseases and conditions that the organoids have been used to model.
Organoids as tools to model cancer
Cancer is a condition caused by mutation in cells that lead to their unregulated proliferation, invasion of surrounding and distant tissues through metastasis. The accumulation of mutations in cancer cells leads to considerable genetic heterogeneity within a tumor (Hanahan and Weinberg 2011). This tumor heterogeneity can also be a result of differences in epigenetic alterations, gene expression profiles, metabolic differences, metastatic potential and rates of proliferation of cells, and is considered the main reason for the failure of conventional cancer therapy (McGranahan and Swanton 2015; Fan et al. 2019). Of all the mutations in the cancer phenotype, the driver mutations cause cancer progression while, the drugs are often targets at passenger mutations that do not cause tumor progression (Stratton et al. 2009).
Models for studying cancer include cell lines, animal models and patient-derived tumor xenografts (PDTX), each of which has certain drawbacks that make it suboptimal for disease modeling (Dutta et al. 2017). Tumoroids are organoids that are derived from patient tumors and recapitulate the genotypic and phenotypic features of individual tumors, promoting personalization of therapy based on a patient’s genetic signatures (Sachs and Clevers 2014).
As proof of this concept, a tumoroid model was used to study pancreatic ductal adenocarcinoma (PDAC). The study used 5 PDAC tumoroid samples and treated them with an epigenetic drug, UNC1999, an inhibitor of histone methyltransferase, enhancer of zeste homolog 1 (EZH2) (H3 lysine 27 trimethylation writer). Out of the 5 tumoroids, the drug was effective against only 3 samples. Genetic analysis of the tumoroids revealed that only those 3 tumoroids retained the H3 lysine 27 trimethylation mark. This is indicative of tumoroids property of retaining the epigenetic signatures of the original tumor (Huang et al. 2015). Patient-derived organoids can also be used to model tumor microenvironments via co-culture with the cells that drive tumor microenvironment. Neal et al. (2018) established a protocol for an air–liquid interface culture of patient-derived organoids with immune and fibroblast components that can be grown from primary tumors. Furthermore, organoid technology, when combined with 4D-micropscopy, can be used to study cancer stem cell behavior in response to drug treatment to predict patient outcomes (Fatehullah et al. 2016). Tumoroids have also been shown to be good models to study hypoxia, oncogenesis, and metastasis (Saglam-Metiner et al. 2019). One of the major advantages of organoids in cancer research is it allows for precise individualized treatment strategies. The organoids from patient-derived healthy and tumor tissues can be comparatively evaluated for efficient drug screening. Certain cancers types such as gastric and gallbladder cancers are reported to have ascended from infectious agents. Co-culturing tumor organoid with infectious agent in vitro would help in understanding the mechanism of pathogenicity. Similarly, virus–cancer interactions; for instance, liver organoids co-cultured with hepatitis virus, or gastric organoids co-cultured with Epstein Barr virus can also be studied (Drost and Clevers 2018).
Jacob et al. (2020) established a glioblastoma organoid biobank from patient tissue and reported that the established organoids maintain parental tumor heterogeneity, mutation and gene expression. These organoids can be used to rapidly test patient-specific treatment strategies (Jacob et al. 2020). Recently, colonic organoids derived from patients with familial adenomatous polyposis (FAP) were used to test potential anticancer activity of XAV939, rapamycin and geneticin, and it was seen that geneticin specifically inhibited the growth of APC-mutant FAP-colonic organoids (Crespo et al. 2017). Numerous diseases, such as Huntington’s disease, Alagille Syndrome and several cancers have been modeled using organoids (Guan et al. 2017; Mehta et al. 2018; Tuveson and Clevers 2019).
Plasticity of cancer cells have not been entirely understood. A 4-Dimensional (4D) live cell imaging of organoids have been utilized to study the tissue dynamics and also lineage tracing. Intravital imaging involves stable fluorescent labeled organoid culture followed by its transplantation in vivo. This is followed by tracing and imaging the primary and metastasized cells of the labeled organoids in vivo up to one year (Rios and Clevers 2018).
Modeling genetic disorders
The property of organoid culture to retain the genetic signatures of the original tissue makes it a reliable method to model genetic disorders. The current strategy for organoids for genetic disorders use either introducing mutations in the wild type organoids which uses CRISPR-Cas9 technology or establishing organoids from patient-derived samples or biopsies.
An example of a genetic disease that has been modeled using organoids is cystic fibrosis (CF). CF is an autosomal recessive condition that is caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (Riordan et al. 1989; Stutts et al. 1995). Over 2000 mutations have been identified within the CFTR gene, which results in a very wide distribution of severity of the disease phenotype. Dekkers et al. (2013) were the first to report the use of intestinal organoids to model CF. CFTR gene is activated by cyclic adenosine monophosphate (cAMP), the level of which is increased by forskolin, an activator of adenylyl cyclase. They developed a forskolin-induced swelling (FIS) assay that could be used to screen drugs against specific mutations of the CFTR gene to create personalized treatment modalities for CF patients. CF patient biobanks are now being developed with organoids generated from intestine, lung, kidney and pancreas (Dekkers et al. 2016; Hohwieler et al. 2017).
Multiple studies have been conducted in modeling and rescue of the retinal organoids in Retinitis pigmentosa (RP) caused due to either X-linked or autosomal mutations. Retinal organoids generated from patient-derived iPSCs have been used to study the tRNA nucleotidyl transferase, CCA-adding 1 (TRNT1) mutant in RP (Perez-Lanzon et al. 2018). In another study of X-linked RP mutation, the RP2 knockout and RP2 mutant patient-derived retinal organoids showed photoreceptor cell death on 150 days which was rescued using an adeno-associated virus mediated gene augmentation (Lane et al. 2020). Use of organoid technology in the repair and replacement of photoreceptors in RP has been detailed by Llonch et al. (2018).
Hereditary multiple intestinal atresia (HMIA) is an autosomal recessive genetic disease caused by mutation in TTC7A gene (tetratricopeptide repeat domain 7A). Intestinal organoids derived from two HMIA patients showed the pathogenicity of TTC7A mutations causing gain of function of differentiation and loss of function of epithelial proliferation (Bigorgne et al. 2014; Perez-Lanzon et al. 2018). Polycystic kidney disease (PKD) is a diverse genetic disease with two types of autosomal dominant and autosomal recessive with both causing formation of cysts in the kidney. Using CRISPR/Cas system, hESCs were gene edited to PKD mutant and kidney organoids were generated (Freedman et al. 2015; Perez-Lanzon et al. 2018). Timothy syndrome, the genetic disease, caused by mutations in calcium voltage-gated channel subunit alpha1 C (CACNA1C) gene, was studied in an assembloid model consisting of subpallium spheroids and cortical spheroids generated from patient-derived iPSCs. There was an observed reduction in saltation length of neurons that held back neuron migration. This was overcome by inhibiting the L-type calcium channel function (Birey et al. 2017).
Alagille syndrome (ALGS) is an autosomal genetic disease affecting multiorgan systems including liver, heart, skeleton and others. The liver organoid model generated from patients was used to study the pathogenicity of the mutant JAG1 gene that encode Notch receptor ligand associated with ALGS. The organoid model study helped in the rescue of impaired ducts on reversal of JAG1 mutation in vitro (Guan et al. 2017).
Modeling infectious diseases
An organoid’s property of retaining the genetic signatures of the original tissue makes it an excellent model to study host-microbe interactions (Bartfeld 2016). Many of the pathogens that infect humans cannot be grown in 2D cultures. Due to advances in 3D cell culture and organoid techniques, microorganisms that were once “unculturable” are now amenable to culture. Examples of such infectious agents include norovirus and rotavirus. Both viruses are now cultured in either 3D or 3D-derived 2D culture systems. Hepatic organoids have been utilized by Baktash et al. (2018) to model Hepatitis C virus (HCV) entry into hepatocytes, demonstrating that HCV interacts with CD81, epidermal growth factor (EGF) receptor and scavenger receptor class B type 1 (SCARB1) and gathers in tight junctions, where it interacts with occludin and claudin-1 and enters the cell through endocytosis mediated by clathrin (Baktash et al. 2018).
A clinically relevant application of organoids in the study of host-microbe interactions is in the elucidation of diseases with high variation between individuals, due to various risk factors such as age, gender, and genetic risk factors. Helicobacter pylori, for example, infects about 50% of the human population, but only a small subset of individuals develops gastric cancer and gastric ulcers (Suerbaum and Michetti 2002). Organoid infected with H. pylori showcased increased proliferation due to oncogenic cagA and increased β-catenin signaling (Fatehullah et al. 2016). Establishing organoid biobanks of patients that developed gastric cancer due to H. pylori infection and studying the variations in gene expression in H. pylori molecular targets could provide a molecular basis for the differential response to H. pylori. Many other GIT infections have been modeled in organoids, including norovirus and rotavirus. Ettayebi et al. (2016) established that ex vivo enteroids support human norovirus infection. Human intestinal enteroids (HIEs) were grown as monolayers and infected with norovirus isolated from stool samples (Ettayebi et al. 2016). Rotavirus was successfully cultured by Saxena et al. (2016) by subjecting HIEs to lysates of MA104 cells infected with rotavirus. The HIE model maintained the virulence and supported replication of both norovirus and rotavirus. Yin et al. (2015) used HIEs to test the efficacy of an antiviral monoclonal antibody, which was found to significantly inhibit rotavirus infection.
3D organoids of the brain have also been used to study the pathogenesis of Zika virus (ZIKV) infection (Cugola et al. 2016; Dang et al. 2016; Qian et al. 2017). Neural organoid models were used to study the effect ZIKV infection had on neurogenesis and growth. Studies have shown that ZIKV infected neural stem cells (NSCs) show mitochondrial swelling, nuclear pyknosis, smooth membrane structures and viral envelops. These signatures were modeled in vitro, and infected organoids showed gene expression profiles that indicated that ZIKV prevented neurosphere formation during fetal development, leading to severe damage of tissues (Camp et al. 2015). Furthermore, ZIKV was found to induce the premature differentiation of infected NPCs and caused defects in the mitotic mechanisms in these cells and upregulate toll-like receptor 3 (TLR3), leading to defects in neurogenesis and eventually, cell death (Dang et al. 2016; Gabriel et al. 2017). Cerebral organoids can be models of neurodevelopmental disorders and have been used to show that previously approved drugs could inhibit zika virus replication and prevent vertical transmission of the virus (Zhou et al. 2017; Trujillo and Muotri 2018).
Kidney and liver organoids have even been used to model SARS-CoV-2 (Zhao et al. 2020; Allison 2020). The pathogenic mechanisms of many other microbes, such as Toxoplasma gondii, Cryptosporidium and Salmonella, have been modeled using organoids (Heo et al. 2018; Yin and Zhou 2018; Delgado Betancourt et al. 2019). Shigatoxigenic Escherichia coli is studied in PSC derived intestinal organoids. Similarly, pathogenicity and multidrug resistance Mycobacterium tuberculosis strain that causes pulmonary tuberculosis disease was evaluated in human lung alveolar organoids derived from adult stem cells. (Iakobachvili and Peters 2017; Li et al. 2020b). Based on the organoid study, cause of the gall bladder carcinoma was reported to be an infection with Salmonella enterica serovar Typhi. Study of other parasites such as Nippostrongylus brasiliensis, Cyclospora sp., Cryptosporidium sp. and Giardia sp. are also being evaluated (Iakobachvili and Peters 2017). Listeria monocytogenes, which causes food borne diarrhea in adults and meningitis in neonates is studied in intestinal organoids derived from fetal tissue. This bacterium rearranges the host cell actin to form a comet like tail formation. Campylobacter jejuni is studied in murine small intestinal organoids and Clostridium difficile is studied in induced human intestinal organoids (Hentschel et al. 2021). Pathophysiology of idiopathic pulmonary fibrosis that causes dyspnea due to fibrotic scarring in the lungs is modeled in lung organoids. Human lung organoids are also used to model respiratory viruses such as human parainfluenza virus 3 and respiratory syncytial virus (RSV) (Li et al. 2020b). Lung bud organoids are used in the modeling of Hermansky–Pudlak syndrome, bronchiolitis and pulmonary fibrosis (Chen et al. 2017).
Modeling neurodegenerative and neuropsychiatric disorders
Organoids provide potentially viable alternative to understand the processes underlying the development and disorders of the human brain. The differentiation of neurons generated in vitro has been the main challenge in developing iPSC models for late-onset neurodegenerative diseases. Recent advances in culturing techniques have allowed for the growth of brain organoids from patients suffering from Alzheimer’s disease (Raja et al. 2016; Gonzalez et al. 2018). Gonzalez et al. (2018) reported the establishment of patient iPSC-derived cerebral organoids to study Alzheimer’s disease (AD). The study reported that the organoids, over time, spontaneously develop the biochemical and morphological traits associated with AD, including structures that very closely resemble amyloid plaques and neurofibrillary tangles (NFTs). These finding were unique to AD patient-derived organoids. Various controls, including iPSCs from healthy individuals, patients with Creutzfeldt-Jakob syndrome, mouse ESCs and mouse iPSCs did not share these cellular signatures (Gonzalez et al. 2018). Organoids have also been established from patients with Huntington’s disease. The phenotypes of neurons generated from patients were commensurate with the characteristics of neurons in vivo. iPSC-derived cortical neurons generated from patients with Huntington’s disease showed variation in gene expression, maturation and morphology, when compared to those derived from healthy individuals (Mehta et al. 2018).
Human organoids recapitulate the early developmental processes that occur in the brain of those affected by autism spectrum disorder (ASD) and could provide insight into its neurobiology (Mariani et al. 2015). Cerebral organoids from patients with ASD showed abnormal proliferation of neuronal progenitor cells and a higher number of GABAergic neurons. This finding supports the hypothesis that ASDs are caused by an imbalance in the excitatory/inhibitory neuronal networks in the brain (Rubenstein 2010). Trevino et al. (2020) recently used brain organoid models to understand the role of epigenetic modifications in the development of ASD. Their method showed that organoid models could be used to study mid-stage neurons as well as late stage (Trevino et al. 2020).
Mutations of the DISC1 gene have been associated with various mood and neurodevelopmental disorders, such as depression, schizophrenia, bipolar disorder and ASD (Porteous et al. 2011; Prytkova and Brennand 2017). However, the precise mechanism by which DISC1 mutations effect development of the brain has been unclear. Studies by Ye et al. (2017) indicated that DISC1 interacts with the protein NDE11 to regulate the attachment of NDE11 to the kinetochore. Mutations in the gene cause a disruption of DISC1/NDE11 complex formation, prolonging mitosis and thus affects cell cycle progression of glial cells (Ye et al. 2017). These findings were also mirrored in organoids established from patients with schizophrenia. Hippocampal dentate gyrus organoids that were established from these patients demonstrated reduced generation of granular neurons, decreased spontaneous neurotransmitter release and reduced neuronal activity, whereas those generated from bipolar patients showed hyperexcitability. The hyperexcitability of neurons generated from bipolar patients could be ameliorated by lithium administration only in those cells generated from patients who responded to lithium treatment (Mertens et al. 2015).
Parkinson’s disease (PD) is caused due to accumulation of Lewy bodies that lead to selective degeneration of dopaminergic neurons. PD can be studied by modeling of midbrain organoids (Smits and Schwamborn 2020; Galet et al. 2020). Animal models used to study the brain development and function in neuropsychiatric diseases fail to match the human brain system. Human brain organoids serve as a better model in such cases. For example, the effect of prenatal drug exposure in neocortical organoids such as cocaine showed premature differentiation and neurodevelopmental defects due CYP3A5-induced reactive oxygen species (ROS) production. This study can be extrapolated to evaluate the targets and effects of drug abuse on brain (Lee et al. 2017; Wang 2018).
Applications in regenerative medicine
One of the major therapeutic applications of organoids is in the field of regenerative medicine. Autologous organoid transplantation is an exciting application for organoids. Organ transplantation therapies carry risks of rejection and shortage of suitable healthy organs can adversely affect patient treatment (Prior et al. 2019). Organoid has been identified as promising alternative to small intestine, liver, kidney, or other tissue’s transplantation (Sato et al. 2009; Huch et al. 2015; Kim et al. 2018; Mansour et al. 2018). ASCs from the healthy region of the affected organ have the potential to differentiate into an organ tissue. This characteristic of ASCs can be used in the repair and transplantation of grafts (Drost and Clevers 2017). Organoids can serve as an alternative for organ transplants in two ways, a) as a replacement in diseases and injuries such as retinal degeneration or intestinal damage and b) as replacement for gene defective organs through gene-corrected organoids (Lancaster and Knoblich 2014).
Kidney organoids developed from patient-derived PSCs have been studied as a potential alternative in kidney transplantation (Grassi et al. 2019). Transplantation of intestinal organoids generated from intestinal stem cells through endoscopy could help patients recover from intestinal bowel disease (Okamoto et al. 2020). Human islet-like organoids generated by Yoshihara and team showed recovery of glucose homeostasis after transplantation in diabetic NOD/SCID mice (Clarke et al. 2018). Engraftment of cerebral organoids into mouse models showed enhanced survival and vascularization compared to transplants of neural progenitor cells. The transplanted organoids contained a large NSC pool and showcased multilineage neurodifferentiation at 2 and 4 weeks post-grafting (Daviaud et al. 2018). Lenti et al. (2019) recently generated lympho-organoids that contained T and B cells which support antigen-specific immunity upon transplantation into mice.
Furthermore, organoid models may play a major role in precision medicine, with diseases affected various organs could be modeled with the patients’ genetic signatures (Lenti et al. 2019). Once the organoids are generated with patients’ genetic signatures, such organoids can be tested for effective drugs for corresponding disease or disorders. This make the treatment module precise and personalized. Ivacaftor drug tested in organoids generated from CF patients, showed promising results and later translating into those patients helped recuperate (Takahashi 2019). Similarly in pancreatic duct cancer, drug screening using organoids generated from patients has been found to be promising in discovering effective drug (Takahashi 2019). Li et al. (2020a, b) has listed a number of cancers where in patient-derived organoids were used in the screening of anticancer drug that are effective and less toxic. In recent years, advances in the translational research has made it possible to generate and cryopreserve organoids from patient-derived tumor and normal cells in organoid banks (Li et al. 2020a).
Lab-on-chip platforms
As organoids begin to grow, the core of each organoids becomes more distant from the nutrient media, causing lack of nutrition. Furthermore, the buildup of waste that is not removed also contributes to death of cells in the core of the organoids (Sailon et al. 2009). These limitations can be addressed using microfluidic platforms, called organs-on-chip or lab-on-chip, to deliver nutrition to the center of each organoid and remove the waste generated at the core. In a study by Karzbrun et al. (2018), the authors used the lab-on-chip brain organoid model to study the wrinkling and folding mechanism during brain development. Brain organoid-on-chip models have also been used to study of effect of prenatal nicotine and cannabis exposure on brain development (Wang et al. 2018; Ao et al. 2020).
Another field in which organ-on-chip models have shown significant potential is toxicology where it has been suggested that these models may help to reduce the number of animals used and to potentially replace animal models (Marx et al. 2016). Existing preclinical models of toxicology are ineffective, with only 12% of candidate drugs from clinical trials reaching the market. Animal models vary in blood flow, transporter expression and plasma protein binding, thus, tubular secretion data cannot be extrapolated to humans. Ex vivo tissues have limited viability with 2D monocultures are viable only for 2 weeks and interindividual variability leads to errors in generalization (Yeung and Himmelfarb 2019). Thus, robust and predictable preclinical models of the renal tubules are required to study secretion, absorption and infection. Sakolish et al. (2018) used a kidney-on-chip models in combination with an in silico model that extrapolates results to in vivo clearance and predicts renal clearance using creatinine (negative control), perfluorooctanoic acid (positive control), gentamycin and cisplatin. Although the model does have drawbacks that need to be addressed, it provides fairly accurate predictions for tubular reabsorption (Sakolish et al. 2018). Wang et al. (2019a) utilized kidney-on-chip to study virus-related kidney dysfunction, using a pseudorabies virus induced kidney disease model. The distal tubular model showed dysregulated Na+ reabsorption, broken reabsorption barriers and transformed microvilli, demonstrating that lab-on-chip platforms can be used to understand the pathophysiology of diseases (Wang et al. 2019a).
Thus, organoid-on-chip models may provide a way through which some of the drawbacks of traditional organoid culture (TOC) can be addressed. TOCs fail to recapitulate the precise microenvironment and its cues for organ development in vivo. One such area where organ-on-chip can help improvise the organoids is in the management of biochemical microenvironment. In TOCs controlling the morphogen gradients at different time points becomes a challenging task. The organ-on-chip model uses a pair of microchannels that can act as a source and sink to precisely control the biochemical environment (Park et al. 2019). This model was use in the development of neural tube patterning where SHH signaling and BMP gradients are formed along the dorsoventral axis (Park et al. 2019). Similarly, this model was used in generating intestine-on-chip by creating a WNT and BMP signaling molecule gradients in the intestinal organoids (Park et al. 2019). Lack of vasculature in the traditional organoid culture cause two problems: a) oxygen supply and b) nutrient supply and waste removal from the inner core of the organoids. Organ-on-chip model with the microfluidic device that has dual microchannels that can serve as an ideal alternative to vasculature found in organs in vivo. Biophysical environment, another challenge in the traditional organoid culture, is overcome using organ-on-chip model. For instance, the peristalsis or the movement of gut can be mimicked using a peristaltic pump attached in a stomach-on-chip model. Yet another use of organ-on-chip platforms is in reducing variability. The automated system help reduce batch to batch variability in size, structure and gene expression patterns (Park et al. 2019).
With advances in these techniques, the future could see highly reproducible lab-on-chip models that could be used for high throughput analysis. Making these systems more uniform will allow for data generated through experiments to be reproducible. With the advent of personalized medicine, the organoid-on-chip models could be used in personalized drug testing in clinical settings. They could also help in clinical trials by abrogating the “false positive” drugs that may get through the screening system due to variations in animal models and monoculture settings. Figure 3 highlights the important applications of patient-derived human organoids in developing personalized medicine.
Application of organoids in evolutionary studies
Brain organoids have been used to understand the changes that occurred during the evolution of man from Denisovan and Neanderthals who are the extant closest relatives to modern humans. One method described by Trujillo et al. (2021) uses introducing a Denisovan/Neanderthal gene variant in the organoids via hPSCs, and observing the morphological, developmental and synaptogenic changes, if any, compared to modern human cortical organoids as controls. The authors first completed a genome wide analysis and identified 61 gene coding variants observed in Denisovan or Neanderthal genomes that carried no substitutions in humans. NOVA1, neuro-oncological ventral antigen 1, one of the candidate gene showed an isoleucine-to-valine single nucleotide substitution at position 200 in archaic genomes (Denisovan/Neanderthal) which was lost in humans during evolution. The hPSCs used for the generation of cortical organoids were CRISPR-Cas9 genome edited and the human NOVA1Hu/Hu gene replaced with the archaic NOVA1Ar/Ar. There was an observed increase in the rigid surface complexity, slower growth, and altered electrophysiological properties in cortical organoids of NOVA1Ar/Ar archaic variant as compared to NOVA1Hu/Hu human cortical organoids. This study revealed the mutations were exclusively found in the modern day humans. The human-specific mutations in NOVA1Hu/Hu not observed in archaic NOVA1Ar/Ar may be due to functional role during the course of evolution. Various researchers, although commended this work, have been skeptical due to two reasons: a) there is a limitation to validate these evolutionary claims in the not well-preserved soft tissues of Neanderthal brains and b) the organoid may not represent the actual Neanderthal brain, moreover, the observed difference in organoid morphology by this single protein change to archaic one would be due to compounding effect (Remmel 2021).
Limitations of organoid systems
Despite the potential of organoids in understanding disease and development, like any other system, there are limitations that need to be addressed. First, although organoids have been demonstrated to mimic the cellular microenvironment of tissues more faithfully than 2D culture systems, there are minute differences in gene expression patterns between organoids and the corresponding in vivo tissues (Pollen et al. 2019). Research is still on to determine the extent to which the organoids can recapitulate the in vivo organ systems (de Souza 2018). Organoid formation should ideally mimic the organ development in its entirety as in the embryo/fetus, but the timing and concentration of various growth factors involved makes it difficult to generate the organoids that match the organs exactly (Kim et al. 2020). Another challenge being that organoids generated from hPSCs, show fetal tissue gene expression profiles, which may not be ideal to study the mature tissue or organ systems (de Souza 2018). Organoids derived from primary tissues can be contaminated by cells from neighboring tissues depending on the sampling method. It has also been reported that 25–95% of tumoroids were shown to be contaminated by “normal” cells (Pauli et al. 2017). Due to the complexities of organoids compared to that of 2D monolayers, tackling the variability could become a tedious task. To start with, the hPSCs used for generating spheroids show variability in their genotypes. Secondly, the batches of organoids from such starting material would show further differences and thirdly this variability also percolates down to multiple organoids of the same batch and within the regions of the same organoids (de Souza 2018). These heterogeneous cultures will contain various clones, with some clones being more predominant than others. Thus, the proportion of cells in an organoid culture harboring a mutation of interest will have to be validated to accurately determine drug response and make experiments reproducible (Huch et al. 2017). These challenges add to further woes with the variability in the growth conditions of different batches that could skew results and reproducibility of experiments (Huch et al. 2017). Furthermore, the lack of vasculature along with immune cells makes it difficult to recapitulate the same conditions as in the tissue/organ systems in vivo (de Souza 2018).
Growth of organoids in Matrigel, a tumor-derived ECM matrix, also impedes their potential clinical application due to the risk of transfer of pathogens and immunogens (Clarke et al. 2018). Growth of organoids in synthetic hydrogels have shown promise in dealing with this limitation, with the same hydrogels used as vehicles to deliver the organoids in mice models (Cruz-Acuña et al. 2018). Generation of organ and tissue specific macro-architecture in organoids remains another challenge that hinders their use in transplantation therapies (Grebenyuk and Ranga 2019). Advances in the development of 3D scaffolds that accurately mimic in vivo tissue architecture will lead to more success in organoid transplantation strategies.
As organoids are found to have more and more relevance and applications, large scale manufacture of organoids becomes inevitable. Due to heterogeneity of cell types, generation of viable organoids with higher yield has been a major challenge. For instance, while generating the intestinal organoids, not all the aggregate of cells bud into organoids. However, by selectively culturing the aggregate of cells that has the potential to develop into organoids, would increase the yield in cultures. Arora et al. (2017) developed a technique of the hind gut cell that can aggregate and can grow into the intestinal organoids. They used an automated capillary-based sorting system where an image processing and analysis unit selects the viable spheroids by identifying them based on the given preset characteristics such as morphology, size, number of cells etc., via a microscope, followed by a three-axis (x, y and z axes) positioning unit that renders micropipette in place along with the spheroid harvesting unit that harvests the potential spheroids for further use.
Ethical challenges in organoid culture are related to the use and derivation of PSCs. Informed consent of the donor in case of patient-derived organoids for further use and limitations of their applications have been considered in the organoid culture research similar to those in developmental biology and clinical research (Munsie et al. 2017).
Conclusion
This review has aimed to collect information on the various strategies employed in organoid culture and to address the potential of this form of cell culture in modeling diseases, as a tool for drug testing, and to better understand the developmental processes involved in the formation of various organs in the human body. The ability of organoids to recapitulate the genetic and epigenetic signatures of the original tissue is of relevance in the field of personalized medicine and can help find therapies against various diseases and conditions. Methods to co-culture tumoroids with tumor environmental cells have allowed the study of tumor-environment interactions, which could possibly lead to a better understanding of the progression of cancer and its potential treatment. The combination of organoid culture with CRISPR/Cas technology and organ-on-chip devices to incorporate and study vasculature are methods that may help better recapitulate in vivo tissue biology. Organoids derived from patients do not deviate in their phenotype, and thus are good models to study disease. Furthermore, organoid biobanks hold the promise of helping us understand the contributions of genetic variations to drug response, helping improve existing therapies and discover new ones. Thus, organoids hold the promise of bridging the gap between animal model systems and human subjects. In the future, this technology may help reduce the use of animals in in vivo drug testing, provide an ethical alternative to animal testing. Use of organoids in evolutionary biology, may additionally serve along with the conventional methods of fossil studies and computational biology.
References
Allende ML, Cook EK, Larman BC et al (2018) Cerebral organoids derived from Sandhoff disease-induced pluripotent stem cells exhibit impaired neurodifferentiation. J Lipid Res 59:550–563. https://doi.org/10.1194/jlr.M081323
Allison SJ (2020) SARS-CoV-2 infection of kidney organoids prevented with soluble human ACE2. Nat Rev Nephrol 16:316–316. https://doi.org/10.1038/s41581-020-0291-8
Amin ND, Paşca SP (2018) Building models of brain disorders with three-dimensional organoids. Neuron 100:389–405. https://doi.org/10.1016/j.neuron.2018.10.007
Angus HCK, Butt AG, Schultz M, Kemp RA (2020) Intestinal organoids as a tool for inflammatory bowel disease research. Front Med 6:1–9. https://doi.org/10.3389/fmed.2019.00334
Antonica F, Kasprzyk DF, Opitz R et al (2012) Generation of functional thyroid from embryonic stem cells. Nature 491:66–71. https://doi.org/10.1038/nature11525
Ao Z, Cai H, Havert DJ et al (2020) One-stop microfluidic assembly of human brain organoids to model prenatal cannabis exposure. Anal Chem 92:4630–4638. https://doi.org/10.1021/acs.analchem.0c00205
Arora N, Alsous JI, Guggenheim JW et al (2017) A process engineering approach to increase organoid yield. Dev 144:1128–1136. https://doi.org/10.1242/dev.142919
Baktash Y, Madhav A, Coller KE, Randall G (2018) Single particle imaging of polarized hepatoma organoids upon hepatitis C virus infection reveals an ordered and sequential entry process. Cell Host Microbe 23:382-394.e5. https://doi.org/10.1016/j.chom.2018.02.005
Barker N, Huch M, Kujala P et al (2010) Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6:25–36. https://doi.org/10.1016/j.stem.2009.11.013
Bartfeld S (2016) Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev Biol 420:262–270. https://doi.org/10.1016/j.ydbio.2016.09.014
Bartfeld S, Clevers H (2015) Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of helicobacter pylori. J vis Exp. https://doi.org/10.3791/53359
Bershteyn M, Nowakowski TJ, Pollen AA et al (2017) Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20:435-449.e4. https://doi.org/10.1016/j.stem.2016.12.007
Betancourt ED, Hamid B, Fabian BT et al (2019) From entry to early dissemination—Toxoplasma gondii’s initial encounter with its host. Front Cell Infect Microbiol 9:1–9. https://doi.org/10.3389/fcimb.2019.00046
Bigorgne AE, Farin HF, Lemoine R et al (2014) TTC7A mutations disrupt intestinal epithelial apicobasal polarity. J Clin Invest 124:328–337. https://doi.org/10.1172/JCI71471
Birey F, Andersen J, Makinson CD et al (2017) Assembly of functionally integrated human forebrain spheroids. Nature 545:54–59. https://doi.org/10.1038/nature22330
Bonnier F, Keating ME, Wróbel TP et al (2015) Cell viability assessment using the Alamar blue assay: a comparison of 2D and 3D cell culture models. Toxicol Vitr 29:124–131. https://doi.org/10.1016/j.tiv.2014.09.014
Broda TR, McCracken KW, Wells JM (2019) Generation of human antral and fundic gastric organoids from pluripotent stem cells. Nat Protoc 14:28–50. https://doi.org/10.1038/s41596-018-0080-z
Cakir B, Xiang Y, Tanaka Y et al (2019) Engineering of human brain organoids with a functional vascular-like system. Nat Methods 16:1169–1175. https://doi.org/10.1038/s41592-019-0586-5
Camp JG, Badsha F, Florio M et al (2015) Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A 112:15672–15677. https://doi.org/10.1073/pnas.1520760112
Cederquist GY, Asciolla JJ, Tchieu J et al (2019) Specification of positional identity in forebrain organoids. Nat Biotechnol 37:436–444. https://doi.org/10.1038/s41587-019-0085-3
Chen YW, Huang SX, De Carvalho ALRT et al (2017) A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19:542–549. https://doi.org/10.1038/ncb3510
Chichagova V, Dorgau B, Felemban M et al (2019) Differentiation of retinal organoids from human pluripotent stem cells. Curr Protoc Stem Cell Biol 50:1–11. https://doi.org/10.1002/cpsc.95
Clarke G, Harley P, Hubber EL et al (2018) Bench to bedside: Current advances in regenerative medicine. Curr Opin Cell Biol 55:59–66. https://doi.org/10.1016/j.ceb.2018.05.006
Cruz-Acuña R, Quirós M, Huang S et al (2018) PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat Protoc 13:2102–2119. https://doi.org/10.1038/s41596-018-0036-3
Cugola FR, Fernandes IR, Russo FB et al (2016) The brazilian zika virus strain causes birth defects in experimental models. Nature 534:267–271. https://doi.org/10.1038/nature18296
D’Amour KA, Agulnick AD, Eliazer S et al (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541. https://doi.org/10.1038/nbt1163
Dang J, Tiwari SK, Lichinchi G et al (2016) Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19:258–265. https://doi.org/10.1016/j.stem.2016.04.014
Danjo T, Eiraku M, Muguruma K et al (2011) Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci 31:1919–1933. https://doi.org/10.1523/JNEUROSCI.5128-10.2011
Daviaud N, Friedel RH, Zou H (2018) Vascularization and engraftment of transplanted human cerebral organoids in mouse cortex. Eneuro 5:1–18. https://doi.org/10.1523/ENEURO.0219-18.2018
de Souza N (2018) Organoids. Nat Methods 15:23–23. https://doi.org/10.1038/nmeth.4576
Dekkers JF, Wiegerinck CL, De Jonge HR et al (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19:939–945. https://doi.org/10.1038/nm.3201
Dekkers JF, Berkers G, Kruisselbrink E et al (2016) Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aad8278
Desai TJ, Brownfield DG, Krasnow MA (2014) Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507:190–194. https://doi.org/10.1038/nature12930
Driehuis E, Gracanin A, Vries RGJ et al (2020) Establishment of pancreatic organoids from normal tissue and tumors. STAR Protoc 1:100192. https://doi.org/10.1016/j.xpro.2020.100192
Drost J, Clevers H (2017) Translational applications of adult stem cell-derived organoids. Dev 144:968–975. https://doi.org/10.1242/dev.140566
Drost J, Clevers H (2018) Organoids in cancer research. Nat Rev Cancer 18:407–418. https://doi.org/10.1038/s41568-018-0007-6
Dutta D, Heo I, Clevers H (2017) Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med 23:393–410. https://doi.org/10.1016/j.molmed.2017.02.007
Dye BR, Hill DR, Ferguson MA et al (2015) In vitro generation of human pluripotent stem cell derived lung organoids. Elife 2015:1–25. https://doi.org/10.7554/eLife.05098
Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12:207–218. https://doi.org/10.1089/adt.2014.573
Eiraku M, Sasai Y (2012) Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr Opin Neurobiol 22:768–777. https://doi.org/10.1016/j.conb.2012.02.005
Eiraku M, Watanabe K, Matsuo-Takasaki M et al (2008) Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3:519–532. https://doi.org/10.1016/j.stem.2008.09.002
Eiraku M, Takata N, Ishibashi H et al (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472:51–58. https://doi.org/10.1038/nature09941
Ettayebi K, Crawford SE, Murakami K et al (2016) Replication of human noroviruses in stem cell-derived human enteroids. Science 353:1387–1393. https://doi.org/10.1126/science.aaf5211
Fan H, Demirci U, Chen P (2019) Emerging organoid models: Leaping forward in cancer research. J Hematol Oncol 12:1–10. https://doi.org/10.1186/s13045-019-0832-4
Fatehullah A, Tan SH, Barker N (2016) Organoids as an in vitro model of human development and disease. Nat Cell Biol 18:246–254. https://doi.org/10.1038/ncb3312
Freedman BS, Brooks CR, Lam AQ et al (2015) Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6:8715. https://doi.org/10.1038/ncomms9715
Fusco P, Parisatto B, Rampazzo E et al (2019) Patient-derived organoids (PDOs) as a novel in vitro model for neuroblastoma tumours. BMC Cancer 19:970. https://doi.org/10.1186/s12885-019-6149-4
Gabriel E, Ramani A, Karow U et al (2017) Recent zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 20:397-406.e5. https://doi.org/10.1016/j.stem.2016.12.005
Galet B, Cheval H, Ravassard P (2020) Patient-derived midbrain organoids to explore the molecular basis of Parkinson’s disease. Front Neurol 11:1005. https://doi.org/10.3389/fneur.2020.01005
Gao ML, Lei XL, Han F et al (2020) Patient-specific retinal organoids recapitulate disease features of late-onset retinitis pigmentosa. Front Cell Dev Biol 8:1–14. https://doi.org/10.3389/fcell.2020.00128
Giacomelli E, Bellin M, Sala L et al (2017) Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Dev 144:1008–1017. https://doi.org/10.1242/dev.143438
Giacomelli E, Meraviglia V, Campostrini G et al (2020) Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell 26:862-879.e11. https://doi.org/10.1016/j.stem.2020.05.004
Gomes AR, Fernandes TG, Vaz SH et al (2020) Modeling Rett syndrome with human patient-specific forebrain organoids. Front Cell Dev Biol 8:1–18. https://doi.org/10.3389/fcell.2020.610427
Gonzalez C, Armijo E, Bravo-Alegria J et al (2018) Modeling amyloid beta and tau pathology in human cerebral organoids. Mol Psychiatry 23:2363–2374. https://doi.org/10.1038/s41380-018-0229-8
Grassi L, Alfonsi R, Francescangeli F et al (2019) Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. https://doi.org/10.1038/s41419-019-1453-0
Grebenyuk S, Ranga A (2019) Engineering organoid vascularization. Front Bioeng Biotechnol 7:1–12. https://doi.org/10.3389/fbioe.2019.00039
Guan Y, Xu D, Garfin PM et al (2017) Human hepatic organoids for the analysis of human genetic diseases. JCI Insight 2:1–17. https://doi.org/10.1172/jci.insight.94954
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Heavner W, Pevny L (2012) Eye development and retinogenesis. Cold Spring Harb Perspect Biol 4:a008391. https://doi.org/10.1101/cshperspect.a008391
Hentschel V, Arnold F, Seufferlein T et al (2021) Enteropathogenic infections: organoids go bacterial. Stem Cells Int. https://doi.org/10.1155/2021/8847804
Heo I, Dutta D, Schaefer DA et al (2018) Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat Microbiol 3:814–823. https://doi.org/10.1038/s41564-018-0177-8
Hinson JT, Chopra A, Nafissi N et al (2015) Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science (80-) 349:982–986. https://doi.org/10.1126/science.aaa5458
Hoang P, Wang J, Conklin BR et al (2018) Generation of spatial-patterned early-developing cardiac organoids using human pluripotent stem cells. Nat Protoc 13:723–737. https://doi.org/10.1038/nprot.2018.006
Hohwieler M, Illing A, Hermann PC et al (2017) Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 66:473–486. https://doi.org/10.1136/gutjnl-2016-312423
Homan KA, Gupta N, Kroll KT et al (2019) Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16:255–262. https://doi.org/10.1038/s41592-019-0325-y
Huang L, Holtzinger A, Jagan I et al (2015) Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med 21:1364–1371. https://doi.org/10.1038/nm.3973
Huch M, Bonfanti P, Boj SF et al (2013) Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32:2708–2721. https://doi.org/10.1038/emboj.2013.204
Huch M, Gehart H, Van Boxtel R et al (2015) Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160:299–312. https://doi.org/10.1016/j.cell.2014.11.050
Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A (2017) The hope and the hype of organoid research. Dev 144:938–941. https://doi.org/10.1242/dev.150201
Hudson J, Titmarsh D, Hidalgo A et al (2012) Primitive cardiac cells from human embryonic stem cells. Stem Cells Dev 21:1513–1523. https://doi.org/10.1089/scd.2011.0254
Hutchinson L, Kirk R (2011) High drug attrition rates - Where are we going wrong? Nat Rev Clin Oncol 8:189–190. https://doi.org/10.1038/nrclinonc.2011.34
Iakobachvili N, Peters PJ (2017) Humans in a dish: The potential of organoids in modeling immunity and infectious diseases. Front Microbiol 8:1–7. https://doi.org/10.3389/fmicb.2017.02402
Iefremova V, Manikakis G, Krefft O et al (2017) An organoid-based model of cortical development identifies non-cell-autonomous defects in Wnt signaling contributing to Miller-Dieker syndrome. Cell Rep 19:50–59. https://doi.org/10.1016/j.celrep.2017.03.047
Jacob F, Salinas RD, Zhang DY et al (2020) A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell 180:188-204.e22. https://doi.org/10.1016/j.cell.2019.11.036
Jain R, Barkauskas CE, Takeda N et al (2015) Plasticity of Hopx+ type I alveolar cells to regenerate type II cells in the lung. Nat Commun 6:6727. https://doi.org/10.1038/ncomms7727
Jeong M, O’Reilly M, Kirkwood NK et al (2018) Generating inner ear organoids containing putative cochlear hair cells from human pluripotent stem cells. Cell Death Dis 9:1–13. https://doi.org/10.1038/s41419-018-0967-1
Jin M, Pomp O, Shinoda T et al (2017) Katanin p80, NuMA and cytoplasmic dynein cooperate to control microtubule dynamics. Sci Rep 7:39902. https://doi.org/10.1038/srep39902
Joseph JS, Malindisa ST, Ntwasa M (2019) Two-Dimensional (2D) and Three-Dimensional (3D) cell culturing in drug discovery. Cell Culture 2:1–22. https://doi.org/10.5772/intechopen.81552
Karzbrun E, Kshirsagar A, Cohen SR et al (2018) Human brain organoids on a chip reveal the physics of folding. Nat Phys 14:515–522. https://doi.org/10.1038/s41567-018-0046-7
Kasendra M, Tovaglieri A, Sontheimer-Phelps A et al (2018) Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci Rep 8:1–14. https://doi.org/10.1038/s41598-018-21201-7
Kim YK, Nam SA, Yang CW (2018) Applications of kidney organoids derived from human pluripotent stem cells. Korean J Intern Med 33:649–659. https://doi.org/10.3904/kjim.2018.198
Kim J, Koo B-K, Knoblich JA (2020) Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 21:571–584. https://doi.org/10.1038/s41580-020-0259-3
Koehler KR, Mikosz AM, Molosh AI et al (2013) Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500:217–221. https://doi.org/10.1038/nature12298
Kondo J, Inoue M (2019) Application of cancer organoid model for drug screening and personalized therapy. Cells 8:470. https://doi.org/10.3390/cells8050470
Kurmann AA, Serra M, Hawkins F et al (2015) Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 17:527–542. https://doi.org/10.1016/j.stem.2015.09.004
Laflamme MA, Chen KY, Naumova AV et al (2007) Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25:1015–1024. https://doi.org/10.1038/nbt1327
Lancaster MA, Knoblich JA (2014) Organogenesisin a dish: Modeling development and disease using organoid technologies. Science 345:1247125–1–1247125–9. https://doi.org/10.1126/science.1247125
Lancaster MA, Renner M, Martin CA et al (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. https://doi.org/10.1038/nature12517
Lane A, Jovanovic K, Shortall C et al (2020) Modeling and rescue of RP2 Retinitis Pigmentosa using iPSC-derived retinal organoids. Stem Cell Reports 15:67–79. https://doi.org/10.1016/j.stemcr.2020.05.007
Laurent LC, Ulitsky I, Slavin I et al (2011) Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8:106–118. https://doi.org/10.1016/j.stem.2010.12.003
Lee C-T, Chen J, Kindberg AA et al (2017) CYP3A5 mediates effects of cocaine on human neocorticogenesis: studies using an in vitro 3D self-organized hPSC model with a single cortex-like unit. Neuropsychopharmacology 42:774–784. https://doi.org/10.1038/npp.2016.156
Lenti E, Bianchessi S, Proulx ST et al (2019) Therapeutic regeneration of lymphatic and immune cell functions upon lympho-organoid transplantation. Stem Cell Rep 12:1260–1268. https://doi.org/10.1016/j.stemcr.2019.04.021
Li Y, Wu Q, Sun X et al (2020b) Organoids as a powerful model for respiratory diseases. Stem Cells Int 2020:1–8. https://doi.org/10.1155/2020/5847876
Li Y, Tang P, Cai S et al (2020a) Organoid based personalized medicine: from bench to bedside. Cell Regen 9:21. https://doi.org/10.1186/s13619-020-00059-z
Llonch S, Carido M, Ader M (2018) Organoid technology for retinal repair. Dev Biol 433:132–143
Loessner D, Stok KS, Lutolf MP et al (2010) Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 31:8494–8506. https://doi.org/10.1016/j.biomaterials.2010.07.064
Longmire TA, Ikonomou L, Hawkins F et al (2012) Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10:398–411. https://doi.org/10.1016/j.stem.2012.01.019
Loomans CJM, Williams Giuliani N, Balak J et al (2018) Expansion of adult human pancreatic tissue yields organoids harboring progenitor cells with endocrine differentiation potential. Stem Cell Reports 10:712–724. https://doi.org/10.1016/j.stemcr.2018.02.005
Ma X, Qu X, Zhu W et al (2016) Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci U S A 113:2206–2211. https://doi.org/10.1073/pnas.1524510113
Mansour AA, Gonçalves JT, Bloyd CW et al (2018) An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 36:432–441. https://doi.org/10.1038/nbt.4127
Mariani J, Coppola G, Zhang P et al (2015) FOXG1-dependent dysregulation of GABA/Glutamate neuron differentiation in autism spectrum disorders. Cell 162:375–390. https://doi.org/10.1016/j.cell.2015.06.034
Marton RM, Miura Y, Sloan SA et al (2019) Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci 22:484–491. https://doi.org/10.1038/s41593-018-0316-9
Marx U, Andersson TB, Bahinski A et al (2016) Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. ALTEX 33:272–321. https://doi.org/10.14573/altex.1603161
Matano M, Date S, Shimokawa M et al (2015) Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med 21:256–262. https://doi.org/10.1038/nm.3802
McCracken KW, Catá EM, Crawford CM et al (2014) Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516:400–404. https://doi.org/10.1038/nature13863
McGranahan N, Swanton C (2015) Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 27:15–26. https://doi.org/10.1016/j.ccell.2014.12.001
Mehta SR, Tom CM, Wang Y et al (2018) Human Huntington’s disease iPSC-derived cortical neurons display altered transcriptomics, morphology, and maturation. Cell Rep 25:1081-1096.e6. https://doi.org/10.1016/j.celrep.2018.09.076
Mertens J, Wang QW, Kim Y et al (2015) Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 527:95–99. https://doi.org/10.1038/nature15526
Miller AJ, Dye BR, Ferrer-Torres D et al (2019) Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc 14:518–540. https://doi.org/10.1038/s41596-018-0104-8
Minoo P, Su G, Drum H et al (1999) Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol 209:60–71. https://doi.org/10.1006/dbio.1999.9234
Miura Y, Pașca SP (2019) Polarizing brain organoids. Nat Biotechnol 37:377–378. https://doi.org/10.1038/s41587-019-0084-4
Morizane R, Bonventre JV (2017) Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat Protoc 12:195–207. https://doi.org/10.1038/nprot.2016.170
Muguruma K, Nishiyama A, Ono Y et al (2010) Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat Neurosci 13:1171–1180. https://doi.org/10.1038/nn.2638
Muguruma K, Nishiyama A, Kawakami H et al (2015) Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep 10:537–550. https://doi.org/10.1016/j.celrep.2014.12.051
Munsie M, Hyun I, Sugarman J (2017) Ethical issues in human organoid and gastruloid research. Dev 144:942–945. https://doi.org/10.1242/dev.140111
Nakano T, Ando S, Takata N et al (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10:771–785. https://doi.org/10.1016/j.stem.2012.05.009
Neal JT, Li X, Zhu J et al (2018) Organoid modeling of the tumor immune microenvironment. Cell 175:1972-1988.e16. https://doi.org/10.1016/j.cell.2018.11.021
Norona LM, Nguyen DG, Gerber DA et al (2016) Modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol Sci 154:354–367. https://doi.org/10.1093/TOXSCI/KFW169
Okamoto R, Shimizu H, Suzuki K et al (2020) Organoid-based regenerative medicine for inflammatory bowel disease. Regen Ther 13:1–6. https://doi.org/10.1016/j.reth.2019.11.004
Park SE, Georgescu A, Huh D (2019) Organoids-on-a-chip. Science 364:960–965. https://doi.org/10.1126/science.aaw7894
Pasca SP (2019) Assembling human brain organoids. Science 363:126–127. https://doi.org/10.1126/science.aau5729
Pasca AM, Sloan SA, Clarke LE et al (2015) Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 12:671–678. https://doi.org/10.1038/nmeth.3415
Pașca AM, Park JY, Shin HW et al (2019) Human 3D cellular model of hypoxic brain injury of prematurity. Nat Med 25:784–791. https://doi.org/10.1038/s41591-019-0436-0
Pauli C, Hopkins BD, Prandi D et al (2017) Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov 7:462–477. https://doi.org/10.1158/2159-8290.CD-16-1154
Perez-Lanzon M, Kroemer G, Maiuri MC (2018) Organoids for modeling genetic diseases, 1st edn. Elsevier Inc, Amsterdam
Pettinato G, Lehoux S, Ramanathan R et al (2019) Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with endothelial cells. Sci Rep 9:1–21. https://doi.org/10.1038/s41598-019-45514-3
Pointon A, Pilling J, Dorval T et al (2017) From the cover: High-throughput imaging of cardiac microtissues for the assessment of cardiac contraction during drug discovery. Toxicol Sci 155:444–457. https://doi.org/10.1093/toxsci/kfw227
Pollen AA, Bhaduri A, Andrews MG et al (2019) Establishing cerebral organoids as models of human-specific brain evolution. Cell 176:743-756.e17. https://doi.org/10.1016/j.cell.2019.01.017
Porteous DJ, Millar JK, Brandon NJ, Sawa A (2011) DISC1 at 10: Connecting psychiatric genetics and neuroscience. Trends Mol Med 17:699–706. https://doi.org/10.1016/j.molmed.2011.09.002
Prior N, Inacio P, Huch M (2019) Liver organoids: from basic research to therapeutic applications. Gut 68:2228–2237. https://doi.org/10.1136/gutjnl-2019-319256
Prytkova I, Brennand KJ (2017) Prospects for modeling abnormal neuronal function in schizophrenia using human induced pluripotent stem cells. Front Cell Neurosci 11:1–8. https://doi.org/10.3389/fncel.2017.00360
Qian X, Nguyen HN, Jacob F et al (2017) Using brain organoids to understand Zika virus-induced microcephaly. Dev 144:952–957. https://doi.org/10.1242/dev.140707
Raja WK, Mungenast AE, Lin YT et al (2016) Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PLoS ONE 11:1–18. https://doi.org/10.1371/journal.pone.0161969
Ravenscroft SM, Pointon A, Williams AW et al (2016) Cardiac non-myocyte cells show enhanced pharmacological function suggestive of contractile maturity in stem cell derived cardiomyocyte microtissues. Toxicol Sci 152:99–112. https://doi.org/10.1093/toxsci/kfw069
Remmel A (2021) Neanderthal-like “mini-brains” created in lab with CRISPR. Nature 590:376–377. https://doi.org/10.1038/d41586-021-00388-2
Richards DJ, Tan Y, Coyle R et al (2016) Nanowires and electrical stimulation synergistically improve functions of hiPSC cardiac spheroids. Nano Lett 16:4670–4678. https://doi.org/10.1021/acs.nanolett.6b02093
Richards DJ, Coyle RC, Tan Y et al (2017) Inspiration from heart development: biomimetic development of functional human cardiac organoids. Biomaterials 142:112–123. https://doi.org/10.1016/j.biomaterials.2017.07.021
Richards DJ, Li Y, Kerr CM et al (2020) Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng 4:446–462. https://doi.org/10.1038/s41551-020-0539-4
Riordan JR, Kerem B, Alon N et al (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066–1073. https://doi.org/10.1126/science.2475911
Rios AC, Clevers H (2018) Imaging organoids: a bright future ahead. Nat Methods 15:24–26. https://doi.org/10.1038/nmeth.4537
Roerink SF, Sasaki N, Lee-Six H et al (2018) Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 556:437–462. https://doi.org/10.1038/s41586-018-0024-3
Ronaldson-Bouchard K, Ma SP, Yeager K et al (2018) Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556:239–243. https://doi.org/10.1038/s41586-018-0016-3
Ronaldson-Bouchard K, Yeager K, Teles D et al (2019) Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nat Protoc 14:2781–2817. https://doi.org/10.1038/s41596-019-0189-8
Ronaldson-Bouchard K, Ma SP, Yeager K et al (2019) Author Correction: Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 572:E16–E17. https://doi.org/10.1038/s41586-019-1415-9
Rubenstein JLR (2010) Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder. Curr Opin Neurol 23:118–123. https://doi.org/10.1097/WCO.0b013e328336eb13
Sachs N, Clevers H (2014) Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev 24:68–73. https://doi.org/10.1016/j.gde.2013.11.012
Saglam-Metiner P, Gulce-Iz S, Biray-Avci C (2019) Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment. Gene 686:203–212. https://doi.org/10.1016/j.gene.2018.11.058
Sailon AM, Allori AC, Davidson EH et al (2009) A novel flow-perfusion bioreactor supports 3D dynamic cell culture. J Biomed Biotechnol 2009:1–7. https://doi.org/10.1155/2009/873816
Saito Y, Onishi N, Takami H et al (2018) Development of a functional thyroid model based on an organoid culture system. Biochem Biophys Res Commun 497:783–789. https://doi.org/10.1016/j.bbrc.2018.02.154
Sakaguchi H, Kadoshima T, Soen M et al (2015) Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun 6:1–11. https://doi.org/10.1038/ncomms9896
Sakolish C, Weber EJ, Kelly EJ et al (2018) Technology transfer of the microphysiological systems: a case study of the human proximal tubule tissue chip. Sci Rep 8:1–14. https://doi.org/10.1038/s41598-018-33099-2
Sato T, Vries RG, Snippert HJ et al (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265. https://doi.org/10.1038/nature07935
Saxena K, Blutt SE, Ettayebi K et al (2016) Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol 90:43–56. https://doi.org/10.1128/jvi.01930-15
Schutgens F, Clevers H (2020) Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol Mech Dis 15:211–234. https://doi.org/10.1146/annurev-pathmechdis-012419-032611
Sloan SA, Andersen J, Pașca AM et al (2018) Generation and assembly of human brain region–specific three-dimensional cultures. Nat Protoc 13:2062–2085. https://doi.org/10.1038/s41596-018-0032-7
Smits LM, Schwamborn JC (2020) Midbrain organoids: A new tool to investigate Parkinson’s disease. Front Cell Dev Biol 8:1–12. https://doi.org/10.3389/fcell.2020.00359
Spence JR, Mayhew CN, Rankin SA et al (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–110. https://doi.org/10.1038/nature09691
Stange DE, Koo B-K, Huch M et al (2013) Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155:357–368. https://doi.org/10.1016/j.cell.2013.09.008
Stevens KR, Pabon L, Muskheli V, Murry CE (2009) Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A 15:1211–1222. https://doi.org/10.1089/ten.tea.2008.0151
Stevens KR, Kreutziger KL, Dupras SK et al (2009) Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci 106:16568–16573. https://doi.org/10.1073/pnas.0908381106
Stratton MR, Campbell PJ, Futreal PA (2009) The cancer genome. Nature 458:719–724. https://doi.org/10.1038/nature07943
Stutts MJ, Canessa CM, Olsen JC et al (1995) CFTR as a cAMP-dependent regulator of sodium channels. Science 269:847–850. https://doi.org/10.1126/science.7543698
Su HL, Muguruma K, Matsuo-Takasaki M et al (2006) Generation of cerebellar neuron precursors from embryonic stem cells. Dev Biol 290:287–296. https://doi.org/10.1016/j.ydbio.2005.11.010
Suerbaum S, Michetti P (2002) Helicobacter pylori infection. N Engl J Med 347:1175–1186. https://doi.org/10.1056/NEJMra020542
Takahashi T (2019) Organoids for drug discovery and personalized medicine. Annu Rev Pharmacol Toxicol 59:447–462. https://doi.org/10.1146/annurev-pharmtox-010818-021108
Takasato M, Er PX, Becroft M et al (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16:118–126. https://doi.org/10.1038/ncb2894
Takasato M, Er PX, Chiu HS, Little MH (2016) Generation of kidney organoids from human pluripotent stem cells. Nat Protoc 11:1681–1692. https://doi.org/10.1038/nprot.2016.098
Takebe T, Sekine K, Enomura M et al (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499:481–484. https://doi.org/10.1038/nature12271
Tiburcy M, Hudson JE, Balfanz P et al (2017) Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 135:1832–1847. https://doi.org/10.1161/CIRCULATIONAHA.116.024145
Trédan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99:1441–1454. https://doi.org/10.1093/jnci/djm135
Trevino AE, Sinnott-Armstrong N, Andersen J et al (2020) Chromatin accessibility dynamics in a model of human forebrain development. Science 367:1–11. https://doi.org/10.1126/science.aay1645
Trujillo CA, Muotri AR (2018) Brain organoids and the study of neurodevelopment. Trends Mol Med 24:982–990. https://doi.org/10.1016/j.molmed.2018.09.005
Trujillo CA, Rice ES, Schaefer NK et al (2021) Reintroduction of the archaic variant of NOVA1 in cortical organoids alters neurodevelopment. Science (80-) 371:eaax2537. https://doi.org/10.1126/science.aax2537
Underhill GH, Khetani SR (2018) Bioengineered liver models for drug testing and cell differentiation studies. Cmgh 5:426-439.e1. https://doi.org/10.1016/j.jcmgh.2017.11.012
Van Den Berg A, Mummery CL, Passier R, Van der Meer AD (2019) Personalised organs-on-chips: functional testing for precision medicine. Lab Chip 19:198–205. https://doi.org/10.1039/c8lc00827b
Vogt N (2021) Assembloids. Nat Methods 18:27–27. https://doi.org/10.1038/s41592-020-01026-x
Völkner M, Zschätzsch M, Rostovskaya M et al (2016) Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Rep 6:525–538. https://doi.org/10.1016/j.stemcr.2016.03.001
Wang H (2018) Modeling neurological diseases with human brain organoids. Front Synaptic Neurosci 10:1–14. https://doi.org/10.3389/fnsyn.2018.00015
Wang Y, Wang L, Zhu Y, Qin J (2018) Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip 18:851–860. https://doi.org/10.1039/c7lc01084b
Wang S, Wang X, Tan Z et al (2019) Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res 29:1009–1026. https://doi.org/10.1038/s41422-019-0242-8
Wang J, Wang C, Xu N et al (2019) A virus-induced kidney disease model based on organ-on-a-chip: pathogenesis exploration of virus-related renal dysfunctions. Biomaterials 219:1–8. https://doi.org/10.1016/j.biomaterials.2019.119367
Wang M, Zhang L, Gage FH (2020) Modeling neuropsychiatric disorders using human induced pluripotent stem cells. Protein Cell 11:45–59. https://doi.org/10.1007/s13238-019-0638-8
Ware MJ, Colbert K, Keshishian V et al (2016) Generation of homogenous three-dimensional pancreatic cancer cell spheroids using an improved hanging drop technique. Tissue Eng - Part C Methods 22:312–321. https://doi.org/10.1089/ten.tec.2015.0280
Watson CL, Mahe MM, Múnera J et al (2014) An in vivo model of human small intestine using pluripotent stem cells. Nat Med 20:1310–1314. https://doi.org/10.1038/nm.3737
Wells MF, Salick MR, Wiskow O et al (2016) Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from zika virus infection. Cell Stem Cell 19:703–708. https://doi.org/10.1016/j.stem.2016.11.011
Wu LJ, Chen ZY, Wang Y et al (2019) Organoids of liver diseases: from bench to bedside. World J Gastroenterol 25:1913–1927. https://doi.org/10.3748/wjg.v25.i16.1913
Xiang Y, Tanaka Y, Cakir B et al (2019) hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell 24:487-497.e7. https://doi.org/10.1016/j.stem.2018.12.015
Xiang Y, Cakir B, Park I-H (2020) Generation of regionally specified human brain organoids resembling thalamus development. STAR Protoc 1:100001. https://doi.org/10.1016/j.xpro.2019.100001
Ye F, Kang E, Yu C et al (2017) DISC1 regulates neurogenesis via modulating kinetochore attachment of Ndel1/Nde1 during mitosis. Neuron 96:1041-1054.e5. https://doi.org/10.1016/j.neuron.2017.10.010
Yeung CK, Himmelfarb J (2019) Kidneys on chips: emerging technology for preclinical drug development. Clin J Am Soc Nephrol 14:144–146. https://doi.org/10.2215/CJN.06690518
Yin Y, Zhou D (2018) Organoid and enteroid modeling of salmonella infection. Front Cell Infect Microbiol 8:1–13. https://doi.org/10.3389/fcimb.2018.00102
Yin Y, Bijvelds M, Dang W et al (2015) Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antiviral Res 123:120–131. https://doi.org/10.1016/j.antiviral.2015.09.010
Yu DX, Di Giorgio FP, Yao J et al (2014) Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Rep 2:295–310. https://doi.org/10.1016/j.stemcr.2014.01.009
Zhao B, Ni C, Gao R et al (2020) Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 11:771–775. https://doi.org/10.1007/s13238-020-00718-6
Zhou K, Wang L, Yu D et al (2017) Molecular and cellular insights into Zika virus-related neuropathies. J Neurovirol 23:341–346. https://doi.org/10.1007/s13365-017-0514-3
Acknowledgements
Authors thank Manipal Academy of Higher Education, Manipal and TIFAC-CORE in Pharmacogenomics for the support. We thank Junior Research Fellowships (JRF through CSIR-UGC NET) provided to Keshava Prasad.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Contributions
AS, KP, KS and AB conceptualized and wrote the review. KP, AB, SC and KS proofread the manuscript. AS, SC, and KP prepared the figures. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shankaran, A., Prasad, K., Chaudhari, S. et al. Advances in development and application of human organoids. 3 Biotech 11, 257 (2021). https://doi.org/10.1007/s13205-021-02815-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02815-7