Abstract
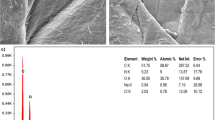
In the present study, the Umbonium vestiarium snail shell (UVS) was used as an abundant and low-cost resource for the removal of Co (II) from aqueous solution. The characteristics of calcined Umbonium vestiarium snail shell (CUVS) were analyzed using FTIR, SEM, MAP, EDAX, and BET analyses. The results showed that the specific surface area of the CUVS was obtained 17.02 m2/g which was an acceptable amount. The presence of Co (II) in the adsorbent structure was confirmed by EDAX, and Map analyses after Co (II) adsorption showed that the adsorbent successfully adsorbed Co (II) from aqueous solution. The effect of different parameters such as, contact time, initial concentration of cobalt ion, the adsorbent dose, and pH value was also investigated. The maximum efficiency of cobalt ion adsorption was measured 93.87% at a pH value of 6, contact time of 80 min, the adsorbent dose of 3 g/L, and initial ion concentration of 10 mg/L. Also, Langmuir, Freundlich, and D–R isotherm models were used to determine the most appropriate isotherm model for cobalt ion adsorption. The adsorption equilibrium data were better fitted with the Langmuir model with a maximum adsorption capacity of 93.46 mg/g. Additionally, the average free energy of adsorption was evaluated in the amount of 1.4085 KJ/mol, revealing a physical adsorption. Moreover, the kinetic behavior study showed that the experimental data follow the pseudo second order kinetic model to the value of correlation coefficient.








Similar content being viewed by others
References
Ahmadpour A, Tahmasbi M, Bastami TR, Besharati JA (2009) Rapid removal of cobalt ion from aqueous solutions by almond green hull. J Hazard Mater 166:925–930
Al-Shahrani S (2014) Treatment of wastewater contaminated with cobalt using Saudi activated bentonite. Alex Eng J 53:205–211
Anwar J, Shafique U, Salman M, Dar A, Anwar S (2010) Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana. Bioresour Technol 101:1752–1755
Bhatnagar A, Minocha A, Sillanpää M (2010) Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem Eng J 48:181–186
Caramalau C, Bulgariu L, Macoveanu M (2009) Adsorption characteristics of Co (II) ions from aqueous solutions on romanian peat moss. Environ Eng Manag J 8:1089–1095
Choi HJ, Yu SW, Kim KH (2016) Efficient use of Mg-modified zeolite in the treatment of aqueous solution contaminated with heavy metal toxic ions. J Taiwan Inst Chem Eng 63:482–489
Cojocaru C, Zakrzewska-Trznadel G, Jaworska A (2009) Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach. Part 1: optimization of complexation conditions. J Hazard Mater 169:599–609
de Almeida FTR, Ferreira BCS, Moreira ALDSL, de Freitas RP, Gil LF, Gurgel LVA (2016) Application of a new bifunctionalized chitosan derivative with zwitterionic characteristics for the adsorption of Cu2+, Co2+, Ni2+, and oxyanions of Cr6+ from aqueous solutions: Kinetic and equilibrium aspects. J Colloid Interface Sci 466:297–309
Dumbauld BR, Ruesink JL, Rumrill SS (2009) The ecological role of bivalve shellfish aquaculture in the estuarine environment: a review with application to oyster and clam culture in West Coast (USA) estuaries. Aquaculture 290:196–223
Esmaeili A, Beni AA (2015) Novel membrane reactor design for heavy-metal removal by alginate nanoparticles. J Ind Eng Chem 26:122–128
Fang F, Kong L, Huang J, Wu S, Zhang K, Wang X, Sun B, Jin Z, Wang J, Huang XJ, Liu J (2014) Removal of cobalt ions from aqueous solution by an amination graphene oxide nanocomposite. J Hazard Mater 270:1–10
Feng N, Guo X, Liang S, Zhu Y, Liu J (2011) Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J Hazard Mater 185:49–54
Foroutan R, Esmaeili H, Rishehri SD, Sadeghzadeh F, Mirahmadi S, Kosarifard M, Ramavandi B (2017a) Zinc, nickel, and cobalt ions removal from aqueous solution and plating plant wastewater by modified Aspergillus flavus biomass: a dataset. Data Brief 12:485–492
Foroutan R, Khoo FS, Ramavandi B, Abbasi S (2017b) Heavy metals removal from synthetic and shipyard wastewater using Phoenix dactylifera activated carbon. Desalin Water Treat 82:146–156
Foroutan R, Esmaeili H, Abbasi M, Rezakazemi M, Mesbah M (2018a) Adsorption behavior of Cu (II) and Co (II) using chemically modified marine algae. Environ Technol 39:2792–2800
Foroutan R, Mohammadi R, Ramavandi B (2018b) Treatment of chromium-laden aqueous solution using CaCl2-modified Sargassum oligocystum biomass: Characteristics, equilibrium, kinetic, and thermodynamic studies. Korean J Chem Eng 35:234–245
Gu P, Zhang S, Li X, Wang X, Wen T, Jehan R, Alsaedi A, Hayat T, Wang X (2018) Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ Pollut 240:493–505
Hadi P, Barford J, McKay G (2013) Synergistic effect in the simultaneous removal of binary cobalt–nickel heavy metals from effluents by a novel e-waste-derived material. Chem Eng J 228:140–146
Javadian H, Vahedian P, Toosi M (2013) Adsorption characteristics of Ni (II) from aqueous solution and industrial wastewater onto Polyaniline/HMS nanocomposite powder. Appl Surf Sci 284:13–22
Khoo FS, Esmaeili H (2018) Synthesis of CaO/Fe3O4 magnetic composite for the removal of Pb (II) and Co (II) from synthetic wastewater. J Serb Chem Soc 83:237–249
Kizilkaya B, Tekinay AA, Dilgin Y (2010) Adsorption and removal of Cu (II) ions from aqueous solution using pretreated fish bones. Desalination 264:37–47
Liao B, Sun WY, Guo N, Ding SL, Su SJ (2016) Comparison of Co2+ adsorption by chitosan and its triethylene-tetramine derivative: performance and mechanism. Carbohydr Polym 151:20–28
Lingamdinne LP, Koduru JR, Roh H, Choi YL, Chang YY, Yang JK (2016) Adsorption removal of Co (II) from waste-water using graphene oxide. Hydrometallurgy 165:90–96
Ma YX, Xing D, Shao WJ, Du XY, La PQ (2017) Preparation of polyamidoamine dendrimers functionalized magnetic graphene oxide for the adsorption of Hg (II) in aqueous solution. J Colloid Interface Sci 505:352–363
Mahini R, Esmaeili H, Foroutan R (2018) Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis. Turk J Biochem 43:623–631
Mittal A, Teotia M, Soni R, Mittal J (2016) Applications of egg shell and egg shell membrane as adsorbents: a review. J Mol Liq 223:376–387
Mousavi SM, Hashemi SA, Esmaeili H, Amani AM, Mojoudi F (2018) Synthesis of Fe3O4 nanoparticles modified by Oak shell for treatment of wastewater containing Ni (II). Acta Chim Slov 65:750–756
Naeimi B, Foroutan R, Ahmadi B, Sadeghzadeh F, Ramavandi B (2018) Pb (II) and Cd (II) removal from aqueous solution, shipyard wastewater, and landfill leachate by modified Rhizopus oryzae biomass. Mater Res Express 5:045501
Ngah WW, Teong L, Toh R, Hanafiah M (2012) Utilization of chitosan–zeolite composite in the removal of Cu (II) from aqueous solution: adsorption, desorption and fixed bed column studies. Chem Eng J 209:46–53
Olu-Owolabi BI, Alabi AH, Unuabonah EI, Diagboya PN, Böhm L, Düring RA (2016) Calcined biomass-modified bentonite clay for removal of aqueous metal ions. J Environ Chem Eng 4:1376–1382
Pawar RR, Bajaj HC, Lee SM (2016) Activated bentonite as a low-cost adsorbent for the removal of Cu (II) and Pb (II) from aqueous solutions: batch and column studies. J Ind Eng Chem 34:213–223
Rangabhashiyam S, Selvaraju N (2015) Adsorptive remediation of hexavalent chromium from synthetic wastewater by a natural and ZnCl2 activated Sterculia guttata shell. J Mol Liq 207:39–49
Sarvestani FS, Esmaeili H, Ramavandi B (2016) Modification of Sargassum angustifolium by molybdate during a facile cultivation for high-rate phosphate removal from wastewater: structural characterization and adsorptive behavior. 3 Biotech 6:251
Smičiklas I, Dimović S, Plećaš I, Mitrić M (2006) Removal of Co2+ from aqueous solutions by hydroxyapatite. Water Res 40:2267–2274
Teimouri A, Esmaeili H, Foroutan R, Ramavandi B (2018) Adsorptive performance of calcined Cardita bicolor for attenuating Hg (II) and As (III) from synthetic and real wastewaters. Korean J Chem Eng 35:479–488
Vilvanathan S, Shanthakumar S (2015) Biosorption of Co (II) ions from aqueous solution using Chrysanthemum indicum: kinetics, equilibrium and thermodynamics. Process Saf Environ 96:98–110
Wang Y, Zhang Y, Hou C, Liu M (2016a) Mussel-inspired synthesis of magnetic polydopamine–chitosan nanoparticles as biosorbent for dyes and metals removal. J Taiwan Inst Chem Eng 61:292–298
Wang Z, Xu J, Hu Y, Zhao H, Zhou J, Liu Y, Lou Z, Xu X (2016b) Functional nanomaterials: study on aqueous Hg (II) adsorption by magnetic Fe3O4@ SiO2—SH nanoparticles. J Taiwan Inst Chem Eng 60:394–402
Wang X, Liu Y, Pang H, Yu S, Ai Y, Ma X, Song G, Hayat T, Alsaedi A, Wang X (2018) Effect of graphene oxide surface modification on the elimination of Co (II) from aqueous solutions. Chem Eng J 344:380–390
Yadav SK, Singh DK, Sinha S (2014) Chemical carbonization of papaya seed originated charcoals for sorption of Pb (II) from aqueous solution. J Environ Chem Eng 2:9–19
Yu H, Pang J, Ai T, Liu L (2016) Biosorption of Cu2+, Co2+ and Ni2+ from aqueous solution by modified corn silk: Equilibrium, kinetics, and thermodynamic studies. J Taiwan Inst Chem Eng 62:21–30
Yu S, Wang X, Pang H, Zhang R, Song W, Fu D, Hayat T, Wang X (2017) Boron nitride-based materials for the removal of pollutants from aqueous solutions: a review. Chem Eng J 333:343–360
Zhang L, Wei J, Zhao X, Li F, Jiang F, Zhang M, Cheng X (2016) Competitive adsorption of strontium and cobalt onto tin antimonate. Chem Eng J 285:679–689
Zhao G, Huang X, Tang Z, Huang Q, Niu F, Wang XK (2018) Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym Chem 9:3562–3582
Zhu T, Row KH (2011) Preparation of amino-modified active carbon cartridges and their use in the extraction of quercetin from Oldenlandia diffusa. J Pharm Biomed Anal 56:713–720
Zhu Y, Hu J, Wang J (2014) Removal of Co2+ from radioactive wastewater by polyvinyl alcohol (PVA)/chitosan magnetic composite. Prog Nuc Energ 71:172–178
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Foroutan, R., Oujifard, A., Papari, F. et al. Calcined Umbonium vestiarium snail shell as an efficient adsorbent for treatment of wastewater containing Co (II). 3 Biotech 9, 78 (2019). https://doi.org/10.1007/s13205-019-1575-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-1575-1