Abstract
Protein p68 is a prototype constituent of DEAD-box protein family, which is involved in RNA metabolism, induced during abiotic stress conditions. In order to address the salinity stress faced by economically important soybean crop, we have transformed soybean cv. PUSA 9712 via direct organogenesis with marker free construct of p68 gene by Agrobacterium-mediated genetic transformation. The putative transgenic plants were screened by Polymerase chain reaction (PCR), Dot blot analysis and Southern blot hybridization. Reverse transcriptase-PCR (RT-PCR) and Quantitative real-time PCR (qRT-PCR) established that the p68 gene expressed in three out of five southern positive (T1) plants. The transformed (T1) soybean plants survived irrigation upto 200 mM of NaCl whereas the non-transformed (NT) plants could not survive even 150 mM NaCl. The transgenic soybean (T1) plants showed a higher accumulation of chlorophyll, proline, CAT, APX, SOD, RWC, DHAR and MDHAR than the NT plants under salinity stress conditions. The transformed (T1) soybean plants also retained a higher net photosynthetic rate, stomatal conductance and CO2 assimilation as compared to NT plants. Further analysis revealed that (T1) soybean plants accumulated higher K+ and lower Na+ levels than NT plants. Yield performance of transformed soybean plants was estimated in the transgenic green house under salinity stress conditions. The transformed (T1) soybean plants expressing the p68 gene were morphologically similar to non-transformed plants and produced 22–24 soybean pods/plant containing 8–9 g (dry weight) of seeds at 200 mM NaCl concentration. The present investigation evidenced the role of the p68 gene against salinity, by enhancing the tolerance towards salinity stress in soybean plants.









Similar content being viewed by others
Abbreviations
- NaCl:
-
Sodium chloride
- CaMV 35S:
-
Cauliflower mosaic virus 35S promoter
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- DHAR:
-
Dehydroascorbate reductase
- MDHAR:
-
Monodehydroascorbate reductase
- MDA:
-
Malondialdehyde
- RWC:
-
Relative water content
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:21–126
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1–15
Arun M, Chinnathambi A, Subramanyam K, Karthik S, Sivanandhan G, Theboral J, Alharbi SA, Kim CK, Ganapathi A (2016) Involvement of exogenous polyamines enhances regeneration and Agrobacterium-mediated genetic transformation in half-seeds of soybean. 3 Biotech 6(2):148
Banu MS, Huda KM, Sahoo RK, Garg B, Tula S, Islam SS, Tuteja R, Tuteja N (2015) Pea p68 imparts salinity stress tolerance in rice by scavenging of ROS-mediated H2O2 and interacts with argonaute. Plant Mol Biol Rep 33(2):221–238
Bartoli CG, Gomez F, Martinez DE, Guiamet JJ (2004) Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot 55:1663–1669
Bates LS, Waldren RP, Teare JD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bauder JW, Brock TA (2001) Irrigation water quality, soil amendment, and crop effects on sodium leaching. Arid Land Res Manag 5(2):101–113
Birt DF, Hendrich S, Anthony M, Alkel DL (2004) Soybeans and the prevention of chronic human disease. In: Specht J, BOerma R (eds) Soybeans: improvement, production and uses, 3rd edn. American society of Agronomy, Madison. pp 1047–1117
Checker VG, Chhibbar AK, Khurana P (2012) Stress-inducible expression of barley Hva1 gene in transgenic mulberry displays enhanced tolerance against drought, salinity and cold stress. Transgenic Res 21(5):939–957
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves:occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Chen N, Liu Y, Liu X, Chai J, Hu Z, Guo G, Liu H (2009) Enhanced tolerance to water deficit and salinity stress in transgenic Lycium barbarum L. plants ectopically expressing ATHK1, an Arabidopsis thaliana histidine kinase gene. Plant Mol Biol Rep 27:321–333
Chen S, Cui X, Chen Y, Gu C, Miao H, Gao H, Chen F, Liu Z, Guan Z, Fang W (2011) CgDREBa transgenic Chrysanthemum confers drought and salinity tolerance. Environ Exp Bot 74:255–260
Conde A, Chaves MM, Geros H (2011) Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol 52:1583–1602
Cuin TA, Betts SA, Chalmandrier R, Shabala S (2008) A root’s ability to retain K+ correlates with salt tolerance in wheat. J Exp Bot 59:2697–2706
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA mini preparation: version II. Plant Mol Biol Rep 1:19–21
Di R, Purcell V, Collins GB, Ghabrial SA (1996) Production of transgenic soybean lines expressing the bean pod mottle virus coat protein precursor gene. Plant Cell Rep 15(10):746–750
Doulis AG, Debian N, Kingston-Smith AH, Foyer CH (1997) Differential localization of antioxidants in maize leaves. Plant Physiol 114:1031–1037
Elsheikh EA, Wood M (1995) Nodulation and N2 fixation by soybean inoculated with salt-tolerant rhizobia or salt-sensitive bradyrhizobia in saline soil. Soil Biol Biochem 27(4–5):657–661
FAOSTAT (2016) Agricultural data. http://www.fao.org/faostat/en#data/QC/visualize. Accessed 3 Aug 2018
Flower DJ, Ludlow MM (1986) Contribution of osmotic adjustment to the dehydration tolerance of water-stressed pigeon pea [Cajanus cajan (L.) Mill sp.] leaves. Plant Cell Environ 9:33–40
Foyer CH, Descourvieres P, Kunert KJ (1994) Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ 17:507–523
Fu XZ, Khan EU, Hu SS, Fan QJ, Liu JH (2011) Overexpression of the betaine aldehyde dehydrogenase gene from Atriplex hortensis enhances salt tolerance in the transgenic trifoliate orange (Poncirus trifoliata L. Raf.). Environ Exp Bot 74:106–113
Gamborg OL, Miller RA, Ojiama K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gao SQ, Chen M, Xia LQ, Xiu HJ, Xu ZS, Li LC, Zhao CP, Chen XG, Ma YZ (2009) A cotton (Gossypium hirsutum) DREbinding transcription factor gene, GhDREB, confers enhanced tolerance to drought, high salt, and freezing stresses in transgenic wheat. Plant Cell Rep 28:301–311
Gendra E, Moreno A, Alba MM, Pages M (2004) Interaction of the plant glycine-rich RNA-binding protein MA16 with a novel nucleolar DEAD box RNA helicase protein from Zea mays. Plant J 38:875–886
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–939
Gill SS, Tajrishi M, Madan M, Tuteja N (2013) A DESD-box helicase functions in salinity stress tolerance by improving photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. PB1). Plant Mol Biol 82:1–22
Guan Q, Wu J, Zhang Y, Jiang C, Liu R, Chai C, Zhu J (2013) A DEAD box RNA helicase is critical for pre-mRNA splicing, cold responsive gene regulation, and cold tolerance in Arabidopsis. Plant Cell 25:342–356
Guan R, Qu Y, Guo Y, Yu L, Liu Y, Jiang J, Chen J, Ren Y, Liu G, Tian L, Jin L (2014) Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J 80(6):937–950
Hanson B, Grattan SR, Fulton A (1999) Agricultural salinity and drainage. University of California Irrigation Program. University of California, Davis
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoi PX, Tuteja N (2012) Identification and sequencing analysis of P68 DEAD-box RNA helicase from Pisum sativum. Nat Sci Technol 28:28–36
Houot V, Etienne P, Petitot AS, Barbier S, Blein JP, Suty L (2001) Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose- dependent manner. J Exp Bot 52:1721–1730
Hu L, Lu H, Liu Q, Chen X, Jiang X (2005) Overexpression of mtlD gene in transgenic Populus tomentosa improves salt tolerance through accumulation of mannitol. Tree Physiol 25:1273–1281
Hu R, Fan C, Li H, Zhang Q, Fu YF (2009) Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol Biol 10:93
Huang Y, Liu ZR (2002) The ATPase, RNA unwinding, and RNA binding activities of recombinant p68 RNA helicase. J Biol Chem 277:12810–12815
Huda KM, Banu MS, Garg B, Tula S, Tuteja R, Tuteja N (2013a) OsACA6, a P-type IIB Ca2+ATPase promotes salinity and drought stress tolerance in tobacco by ROS scavenging and enhancing the expression of stress-responsive genes. Plant J 76:997–1015
Husaini AM, Abdin MZ (2008) Development of transgenic strawberry (Fragaria × ananassa Dutch.) plants tolerant to salt stress. Plant Sci 174:446–455
Khan MS, Ahmad D, Khan MA (2015) Utilization of genes encoding osmoprotectants in transgenic plants for enhanced abiotic stress tolerance. Electron J Biotechnol 18(4):257–266
Li D, Zhang H, Wang X, Song F (2008) OsBIRH1, a DEAD box RNA helicase with functions in modulating defence responses against pathogen infection and oxidative stress. J Exp Bot 59:2133–2146
Light GG, Mahan JR, Roxas VP, Allen RD (2005) Transgenic cotton (Gossypium hirsutum L.) seedlings expressing a tobacco glutathione S-transferase fail to provide improved stress tolerance. Planta 222:346–354
Linder P, Lasko PF, Ashburner M, Leroy P, Nielsen PJ, Nishi K, Schneir J, Slonimiski PP (1989) Birth of DEAD-box. Nature 337:121–122
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ∆∆CT method. Methods 25:402–408
Maas EV, Hoffman GJ (1977) Crop salt tolerance–current assessment. J Irrig Drain Div 103(2):115–134
Maghuly F, Leopold S, Camara Machado AD, Fernandez EB, Khan MA, Gambino G, Gribaudo I, Schartl A, Laimer M (2006) Molecular characterization of grapevine plants transformed with GFLV resistance genes:II. Plant Cell Rep 25:546–553
Malatrasi M, Close TJ, Marmiroli N (2002) Identification and mapping of a putative stress response regulator gene in barley. Plant Mol Biol 50:143–152
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
McCue P, Shetty K (2004) Health benefits of soy isoflavonoids and strategies for enhancement: a review. Crit Rev Food Sci Nutr 44(5):361–367
Meloni DA, Gulotta MR, Martínez CA, Oliva MA (2004) The effects of salt stress on growth, nitrate reduction and proline and glycinebetaine accumulation in Prosopis alba. Braz J Plant Physiol 16(1):39–46
Miyake C, Asada K (1992) Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in the thylakoids. Plant Cell Physiol 35:539–549
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25(2):239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Munns R, James RA, Lauchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043
Munns R, Wallace PA, Teakle NL, Colmer TD (2010) Measuring soluble ion concentrations (Na+, K+, Cl−) in salt-treated plants. In: Sunkar R (ed) Methods in molecular biology Plant stress tolerance: methods and protocols, vol 639. Humana Press Springer, New York, pp 371–382
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol 49:249–279
Okanami M, Meshi T, Iwabuchi M (1998) Characterization of a DEAD box ATPase/RNA helicase protein of Arabidopsis thaliana. Nucleic Acids Res 26:2638–2643
Parkhi V, Kumar V, Sunilkumar G, Campbell LAM, Singh NK, Rathore KS (2009) Expression of apoplastically secreted tobacco osmotin in cotton confers drought tolerance. Mol Breed 23:625–639
Pasapula V, Shen G, Kuppu S, Paez-Valencia J, Mendoza M, Hou P, Chen J, Qiu X, Zhu L, Zhang X, Auld D, Blumwald E, Zhang H, Gaxiola R, Payton P (2011) Expression of an Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought-and salt tolerance and increases fiber yield in the field conditions. Plant Biotechnol J 9:88–99
Pradhan A, Chauhan VS, Tuteja R (2005b) Plasmodium falciparum DNA helicase 60 is a schizont stage specific, bipolar and dual helicase stimulated by PKC phosphorylation. Mol Biochem Parasitol 144:133–141
Rampino P, Pataleo S, Gerardi C, Mita G, Perrotta C (2006) Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ 29:2143–2152
Roychowdhury A, Roy C, Sengupta DN (2007) Transgenic tobacco plants overexpressing the heterologous lea gene Rab 16a from rice during high salt and water deficit display enhanced tolerance to salinity stress. Plant Cell Rep 26:1839–1859
Sanan-Mishra N, Pham XH, Sopory SK, Tuteja N (2005) Pea DNA helicase 45 overexpression in tobacco confers high salinity tolerance without affecting yield. Proc Natl Acad Sci USA 102:509–514
Shabala S (2009) Salinity and programmed cell death: unraveling mechanisms for ion specific signalling. J Exp Bot 60:709–712
Shah K, Singh M, Rai AC (2013) Effect of heat-shock induced oxidative stress is suppressed in BcZAT12 expressing drought tolerant tomato. Phytochemistry 95:109–117
Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104:169–174
Singh SK, Sharma HC, Goswami AM, Datta SP, Singh SP (2000) In vitro growth and leaf composition of grapevine cultivars as affected by sodium chloride. Biol Plant 43:283–286
Soystats (2011) American Soybean Association. http://soystats.com/archives/2011/page_06.htm. Accessed 3 Aug 2018
Subramanyam K, Sailaja KV, Subramanyam K, Muralidhara Rao D, Lakshmidevi K (2011) Ectopic expression of an osmotin gene leads to enhanced salt tolerance in transgenic chilli pepper (Capsicum annum L.). Plant Cell Tissue Organ Cult 105:181–192
Subramanyam K, Arun M, Mariashibu TS, Theboral J, Rajesh M, Singh NK, Manickavasagam M, Ganapathi A (2012) Overexpression of tobacco osmotin (Tbosm) in soybean conferred resistance to salinity stress and fungal infections. Planta 236(6):1909–1925
Talame V, Ozturk NZ, Bohnert HJ, Tuberosa R (2007) The dynamics of water loss affects the expression of drought-related genes in barley. J Exp Bot 58:229–240
Tambussi EA, Bartoli CG, Beltrano J, Guiamet JJ, Araus JL (2000) Oxidative damage to thylakoid proteins in water-stressed leaves of wheat (Triticum aestivum). Physiol Plant 108:398–404
Turkan I, Demiral T (2009) Recent developments in understanding salinity tolerance. Environ Exp Bot 67(1):2–9
Turner NC (1981) Techniques and experimental approaches for the measurement of plant water stress. Plant Soil 58:339–366
Tuteja N (2003) Plant DNA helicases: the long unwinding road. J Exp Bot 54(391):2201–2214
Tuteja R, Pradhan A (2006) Unraveling the ‘DEAD-box’ helicases of Plasmodium falciparum. Gene 376:1–12
Tuteja N, Tuteja R (2004a) Prokaryotic and eukaryotic DNA helicases. Essential molecular motor proteins for cellular machinery. Eur J Biochem 271:1835–1848
Tuteja N, Tuteja R (2004b) Unraveling DNA helicases. Motif, structure, mechanism and function. Eur J Biochem 271:1849–1863
Tuteja N, Sahoo RK, Garg B, Tuteja R (2013) OsSUV3 dual helicase functions in salinity stress tolerance by maintaining photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. IR64). Plant J 76:115–127
Tuteja N, Banu MS, Huda KM, Gill SS, Jain P, Pham XH, Tuteja R (2014) Pea p68, a DEAD-box helicase, provides salinity stress tolerance in transgenic tobacco by reducing oxidative stress and improving photosynthesis machinery. PLoS One 9(5):e98287
USDA (2018) Food composition database. https://ndb.nal.usda.gov/ndb/search/list?home=true. Accessed 3 Aug 2018
Vashisht AA, Pradhan A, Tuteja R, Tuteja N (2005) Cold- and salinity stress-induced bipolar pea DNA helicase 47 is involved in protein synthesis and stimulated by phosphorylation with protein kinase C. Plant J 44:76–87
Velikova V, Yordanov J, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain treated bean plants. Protective role of exogenous polyamines. Plant Sci 151:59–66
Wang Y, Jiang L, Chen J, Tao L, An Y, Cai H, Guo C (2018) Overexpression of the alfalfa WRKY11 gene enhances salt tolerance in soybean. PLoS One 13(2):e0192382
Xu GY, Rocha PSCF, Wang ML, Xu ML, Cui YC, Li LY, Zhu YX, Xia X (2011) A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234:47–59
Zhang HW, Huang ZJ, Xie BY, Chen Q, Tian X, Zhang XL, Zhang HB, Lu XY, Huang DF, Huang RF (2004) The ethylene, jasmonate, abscisic acid and NaCl-responsive tomato transcription factor JERF1 modulates expression of GCC box containing genes and salt tolerance in tobacco. Planta 220:262–270
Zhang WJ, Yang SS, Shen XY, Jin YS, Zhao HJ, Wang T (2009) The salt-tolerance gene rstB can be used as a selectable marker in plant genetic transformation. Mol Breed 23:269–277
Acknowledgements
Jawaharlal Nehru Scholarship (Ref no: SU-1/88/2016-17/79) for doctoral studies awarded by Jawaharlal Nehru Memorial Fund, New Delhi, India is thankfully acknowledged by the first author (Sivabalan Karthik).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13205_2018_1553_MOESM1_ESM.docx
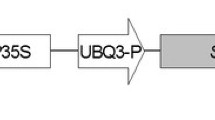
Supplementary Fig. 1 Schematic representation of binary vector pCAMBIA1300 with pea p68 gene (marker free construct). (DOCX 325 KB)
Rights and permissions
About this article
Cite this article
Karthik, S., Tuteja, N., Ganapathi, A. et al. Pea p68, a DEAD-box helicase, enhances salt tolerance in marker-free transgenic plants of soybean [Glycine max (L.) Merrill]. 3 Biotech 9, 10 (2019). https://doi.org/10.1007/s13205-018-1553-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1553-z