Abstract
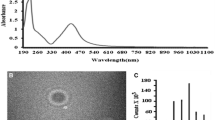
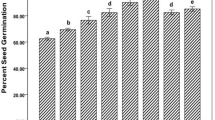
In this study, the interaction of biosynthesized silver nanoparticles (BSNP) with native soil via plant transport was assessed in model pathosystem of Arabidopsis thaliana and Alternaria brassicicola. Foliar application of 5 μg/mL of BSNP reduced number of spores of fungi to 2.2 × 105 from 7 × 105, while numbers of lesions got reduced to 0.9/leaf in treated plants compared to 2.9/leaf in pathogen-infected plant without altering soil pH, electric conductivity, soil organic carbon and soil microbial biomass carbon. Soil enzyme activities including dehydrogenase, acid and alkaline phosphatase, urease, β-glucosidase and protease did not alter significantly in BSNP-treated plants compared to control plants. Application of BSNP did not alter the number of cultivable bacteria, fungi and actinomycetes. Effect of BSNP on uncultured bacterial diversity was measured by DGGE analysis which revealed similar banding pattern in all different treatments except in A. brassicicola-infected (AB) and A. brassicicola-infected plants treated with silver nanoparticles (AB + BSNP) after 120 days. Although AB-infected plants exhibited a decrease in bacterial diversity, treatment of AB + BSNP after 120 days demonstrated maximum bacterial diversity. McIntosh, Shannon, and Simpson diversity indices were calculated based on carbon source utilization pattern by BIOLOG analysis, revealing no significant difference among all treatments in different time intervals. BSNPs have the potential to act as strong antimicrobial agent for plant disease management without altering the native soil microflora.






Similar content being viewed by others
References
Balakumaran MD, Ramachandran R, Balashanmugam P, Mukeshkumar DJ, Kalaichelvan PT (2016) Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol Res 182:8–20
Balashanmugam P, Balakumaran MD, Murugan R, Dhanapal K, Kalaichelvan PT (2016) Phytogenic synthesis of silver nanoparticles, optimization and evaluation of in vitro antifungal activity against human and plant pathogens. Microbiol Res 192:52–64
Berry TD, Clavijo AP, Zhao Y, Jafvert CT, Turco RF, Filley TR (2016) Soil microbial response to photo-degraded C60 fullerenes. Env Poll 21:338e345
Dick WA, Cheng L, Wang P (2000) Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol Biochem 32:1915–1919
Dinesh R, Anandaraj M, Srinivasan V, Hamza S (2012) Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma 173:19–27
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28:213–221
Girilal M, Krishnakumar V, Poornima P, Fayaz AM, Kalaichelvan PT (2015) A comparative study on biologically and chemically synthesized silver nanoparticles induced Heat Shock Proteins on fresh water fish Oreochromis niloticus. Chemosphere 139:461–468
Hänschand M, Emmerling C (2010) Effects of silver nanoparticles on the microbiota and enzyme activity in soil. J Plant Nutr Soil Sci 173:554–558
He S, Feng Y, Ni J, Sun Y, Xue L, Feng Y, Yu Y, Lin X, Yang L (2016) Different responses of soil microbial metabolic activity to silver and iron oxide nanoparticles. Chemosphere 147:195–202
Hosseini F, Mosaddeghi MR, Hajabbasi MA, Mamedov AI (2017) Effects of endophyte-infected and non-infected tall fescue residues on aggregate stability in four texturally different soils. Geoderma 285:195–205
Ignatova LV, Brazhnikov YV, Berzhanova RZ, Mukasheva TD (2015) Plant growth-promoting and antifungal activity of yeasts from dark chestnut soil. Microbiol Res 175:78–83
Janvier C, Villeneuve F, Alabouvette C, Edel-Hermann V, Mateille T, Steinberg C (2007) Soil health through soil disease suppression: which strategy from descriptors to indicators. Soil Biol Biochem 39(1):1–23
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fert Soil 6:68–72
Khan N, Mishra A, Nautiyal CS (2012) Paenibacillus lentimorbus B-30488r controls early blight disease in tomato by inducing host resistance associated gene expression and inhibiting Alternaria solani. Biol Control 62:65–74
Khodakovskaya MV, De Silva K, Biris AS, Dervishi E, Villagarcia H (2012) Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 6(3):2128–2135
Klitzke S, Metreveli G, Peters A, Schaumann GE, Lang F (2015) The fate of silver nanoparticles in soil solution—sorption of solutes and aggregation. Sci Total Environ 535:54–60
Kumar N, Palmer GR, Shah V, Walker VK (2014) The effect of silver nanoparticles on seasonal change in Arctic tundra bacterial and fungal assemblages. PLoS One 9(6):e99953
Kumari M, Mishra A, Pandey S, Singh SP, Chaudhry V, Mudiam MKR, Shukla S, Kakkar P, Nautiyal CS (2016) Physico-Chemical condition optimization during biosynthesis lead to development of improved and catalytically efficient gold nanoparticles. Sci Rep 6:27575
Kumari M, Pandey S, Giri VP, Bhattacharya A, Shukla R, Mishra A, Nautiyal CS (2017a) Tailoring shape and size of biogenic silver nanoparticles to enhance antimicrobial efficacy against MDR bacteria. Microb Pathog 105:346–355
Kumari M, Shukla S, Pandey S, Giri VP, Bhatia A, Tripathi T, Kakkar P, Nautiyal CS, Mishra A (2017b) Enhanced cellular internalization: a bactericidal mechanism more relative to biogenic nanoparticles than chemical counterparts. ACS Appl Mat Intefaces 9:4519–4533
Ladd JN, Butler JHA (1972) Short-term assays of soil proteolytic enzyme activities using proteins and di-peptide derivatives as substrates. Soil Biol Biochem 4:19–30
Levard C, Hotze EM, Lowry GV, Brown GE (2013) Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol 46(13):6900–6914
Maliszewska I (2016) Effects of the biogenic gold nanoparticles on microbial community structure and activities. Ann Microbiol 66:785–794
Mishra A, Nautiyal CS (2009) Functional diversity of the microbial community in the rhizosphere of chickpea grown in diesel fuel-spiked soil amended with Trichoderma reesei using sole-carbon-source utilization profiles. World J Microbiol Biotechnol 25:1175–1180
Mishra S, Singh HB (2014) Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: exploring their scope and potential in agriculture. Appl Microbiol Biotechnol 99(3):1097–1107
Mishra A, Kumari M, Pandey S, Chaudhry V, Gupta KC, Nautiyal CS (2014) Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Biores Technol 166:235–242
Naseby DC, Pascual JA, Lynch JM (2000) Effect of biocontrol strains of Trichoderma on plant growth, Pythium ultimum populations, soil microbial communities and soil enzyme activities. J Appl Microbiol 88:161–169
Nelson DW, Sommers LE (1996) Methods of soil analysis. Part 3. Chemical methods. Soil Science Society of America, American Society of Agronomy, Madison, pp 961–1010
Ocsoy I, Paret ML, Ocsoy MA, Kunwar S, Chen T, You M, Tan W (2013) Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano 7(10):8972–8980
Peyrot C, Wilkinson KJ, Desrosiers M, Sauvé S (2014) Effects of silver nanoparticles on soil enzyme activities with. and without added organic matter. Environ Toxicol Chem 33(1):115–125
Rahmatpour S, Shirvani M, Mosaddeghi MR, Nourbakhsh F, Bazarganipour M (2017) Dose–response effects of silver nanoparticles and silver nitrate on microbial and enzyme activities in calcareous soils. Geoderma 285:313–322
Rejsek K, Formanek P, Pavelka M (2008) Estimation of protease activity in soils at low temperatures by casein amendment and with substitution of buffer by demineralised water. Amino Acids 35:411–417
Sardans J, Penuelas J (2005) Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol Biochem 37:455–461
Schlich K, Hund-Rinke K (2015) Influence of soil properties on the effect of silver nanomaterials on microbial activity in five soils. Environ Poll 196:321–330
Sillen WMA, Thijs S, Abbamondi RG, Janssen J, Weyens N, White JC, Vangronsveld J (2015) Effects of silver nanoparticles on soil microorganisms and maize biomass are linked in the rhizosphere. Soil Biol Biochem 91:14e2
Singh AK, Rai A, Singh N (2016) Effect of long term land use systems on fractions of glomalin and soil organic carbon in the Indo-Gangetic plain. Geoderma 277:41–50
Srivastava PK, Gupta M, Pandey A, Pandey V, Singh N, Tewari SK (2014) Effects of sodicity induced changes in soil physical properties on paddy root growth. Plant Soil Environ 60:165–169
Tabatabai MA (1994) Soil enzymes. In: Mickelson SH, Bigham JM (eds) Methods of soil analysis, part 2. Microbiological and biochemical properties. SSSA, Madison
Tan Y, Cui Y, Li H, Kuang A, Li X, Wei Y, Ji X (2017) Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiol Res 194:10–19
Turner BL, Hopkins DW, Haygarth PM, Ostlec N (2002) β-Glucosidase activity in pasture soils. Appl Soil Ecol 20(2):157–162
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang Z, Zhang L, Zhao J, Xing B (2016) Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ Sci Nano 3:240–255
Wolińska A, Stępniewska Z (2012) Activity in the soil environment “dehydrogenases”. In: Canuto RA (ed) Dehydrogenase. Intech publishing group, Croatia, pp 183–210
Xiu-Mei L, Qi L, Wen-Ju L, Yong J (2008) Distribution of soil enzyme activities and microbial biomass along a latitudinal gradient in farmlands of Songliao plain, Northeast China. Pedosphere 18(4):431–440
Acknowledgements
This study was partially funded by network project of Council of Scientific and Industrial Research (CSIR) “Root SF BSC0204”. MK thanks CSIR for awarding her Senior Research Fellowship (SRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors do not declare any conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumari, M., Pandey, S., Mishra, S.K. et al. Effect of biosynthesized silver nanoparticles on native soil microflora via plant transport during plant–pathogen–nanoparticles interaction. 3 Biotech 7, 345 (2017). https://doi.org/10.1007/s13205-017-0988-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0988-y