Abstract
Polygalacturonases (PG) represent an important member of pectinases group of enzymes with immense industrial applications. A fungal strain Aspergillus niger MTCC478 was used for the production of polygalacturonase both under submerged and solid-state fermentation condition. Further its production was optimized under solid-state fermentation condition with media comprising of wheat bran and tea extract. Purification of an exo-PG was achieved by acetone precipitation (60–90%) and CM-cellulose column chromatography revealing 15.28-fold purification with a specific activity of 33.47 U/mg protein and 1.2% yield. A relative molecular mass of purified PG was approximately 124.0 kDa. The pH and temperature optimum was found to be 4 and 50 °C, respectively. The k cat and K m value for degradation of PGA by the purified enzyme was found to be 194 s−1 and 2.3 mg/mL, respectively. Cu2+ was found to enhance the PG activity while Ag+ completely inhibited the enzyme activity. The application of the purified PG in orange juice clarification was elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pectin, an acidic polysaccharide whose basic structural repeats are α-1,4-linked-d-galacturonic acid, is widely found in the middle lamella and primary cell wall of plants. As an important renewable resource, pectin has great potential applications in the biomedical, food, agricultural, and other industries (Mohnen 2008). The degradation of pectin is mainly facilitated by pectinases group of enzymes, including pectin methyl esterases (PME, E.C. 3.1.1.11), pectin lyases (PL, E.C. 4.2.2.10), exopolygalacturonase (exo-PG, E.C. 3.2.1.67), and endopolygalacturonase (endo-PG, E.C. 3.2.2.15) (Yadav et al. 2009).
Polygalacturonases are pectin-degrading proteins that catalyze hydrolysis of α-1, 4-glycosidic linkages in pectate or other galacturonans at the end, exo-PG (EC 3.2.1.67) or randomly in the middle, endo-PG (EC 3.2.1.15) of polymeric chains. Polygalacturonases (PGs) are produced by various organisms, such as plants (Bird et al. 1988; Hadfield and Benett 1998), bacteria (Jayani et al. 2010; Tariq and Latif 2012; Chen et al. 2014) and fungi (Martins et al. 2013; Ortega et al. 2014; Cheng et al. 2016).
In general, both submerged state fermentation (SmF) and solid-state fermentation (SSF) have been successfully used in pectinases production from different microbial strains (Castilho et al. 2000; Silva et al. 2002; Pedrolli and Carmona 2009; Dinu et al. 2007). The solid-state fermentation is preferred over submerged fermentation based on the fact that it uses various agro-industrial by-products such as soy, pulps of apple, sugar beet and coffee, peels of lemon, oranges and tomato, pomace of apple and citrus fruits, sugarcane bagasse, wheat bran, etc., making the entire process cost effective (Castilho et al. 2000; Yadav and Shastri 2007; Lara-Marquez et al. 2011; Yadav et al. 2014).
Enzymes produced from the fungi Aspergillus, Rhizopus and Penicillium are generally regarded as safe (GRAS) and produce extracellular enzymes which can be easily recovered (Mrudula and Anitharaj 2011). In vegetable and fruit juice industry, juices with high clarity and low viscosity have high commercial values. Fruit generally contains pectin and other polysaccharides leading to fouling and colloid formation and substantially influences the commercial value of juices. Acidophilic pectinases can degrade pectin resulting in viscosity reduction and cluster formation, which facilitates separation through centrifugation or filtration. As a result, the juice attains higher clarity and flavor.
Microbial polygalacturonases have been shown to play role in viscosity reduction and clarification of juice (Kashyap et al. 2001; Kant et al. 2013; Barman et al. 2015; Amin et al. 2017). Acidophilic PGs with optimum pH close to pH of fruit juices are preferably utilized for juice preparation and clarification. Commercial pectinases from Aspergillus niger (Perrone et al. 2006) and Bacillus subtilis (Takao et al. 2000) have been predominantly used as enhancers of juice clarity, color and yield (Oszmianski et al. 2009).
The production, purification and biochemical characterization of polygalacturonase from a fungal strain Aspergillus niger MTCC 478 and its application in fruit juice clarification is reported in the present manuscript.
Materials and methods
Chemicals
Polygalacturonic acid (PGA) and CM-cellulose were purchased from Sigma Chemical Company (St. Louis, MO, USA). Rest of the chemicals were procured either from Merck (Navi Mumbai, India) or S.D. Fine (Mumbai, India) and were used without further purification.
Organism and growth condition
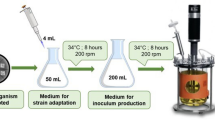
The fungal strain Aspergillus niger MTCC 478 was procured from Microbial Type Culture Collection and Gene Bank, Institute of Microbial Technology, Chandigarh (India) and screened for pectinase production by plate assay method (Molina et al. 2001). The culture was maintained by cultivation on Czapek-Dox agar slants at 26 °C. Based on prominent halo zone formation, the culture was further screened for PG production both under submerged and solid-state fermentation conditions. Submerged fermentation media (M1) comprised (NH4)2SO4 2 g/L, K2HPO4 2 g/L, KH2PO4 2 g/L, Yeast extract 3 g/L, pectin 5 g/L, pH 7.0. The inoculum (4% v/v) was transferred to 25 mL production media in 250-mL Erlenmeyer flask. Cultures were kept in an incubator shaker at 26 °C and 150 rpm. Two different media were used for solid-state fermentation. One of the production mediums (M2) consisted of wheat bran 5.0 g and 5 mL of salt solution. The other medium (M3) consisted of wheat bran 4.5 g, tea extract 0.5 g and 5 mL salt solution. The composition of salt solution was (4 g/L each of K2HPO4, KH2PO4, and NH4SO4) and final moisture content was kept 50%. The inoculum (10% v/w) was transferred to the production media in 250-mL Erlenmeyer flask. Flasks were incubated at 26 °C and cultures were allowed to grow under stationary condition.
Production of polygalacturonase
The fungal strain Aspergillus niger MTCC 478 was grown in ten 250-mL Erlenmeyer flasks containing M3 media. The media was inoculated with 1 mL of spore suspension (5 × 106 spores/mL) in each flask and the flasks were kept at 26 °C in a biological oxygen demand incubator (BOD). On the day of maximum enzyme production, 15 mL of cold distilled water was added in each flask and mixed properly with the help of glass rod. All the cultures were pooled and filtered with four layers of cheese cloth; the filtrate was centrifuged at 10,000 rpm for 20 min at 4 °C. The pellet was discarded and supernatant was used as crude enzyme sources.
Polygalacturonase assay and protein estimation
Enzyme activity of PG was assayed by determining the liberated reducing-end products by standard method (Miller 1959). The reaction solution (2 mL) consisted of 0.5 mL of 1% PGA, 1.4 mL of 100 mM citrate–phosphate buffer (pH 4.0) and 0.1 mL of enzyme solution. It was incubated for 20 min at 37 °C in a water bath. Three mL of dinitrosalicylic acid (DNSA) reagent was added and volume was made 6 mL by addition of 1 mL of distilled water. The solution was boiled for 10 min in a water bath, cooled and absorbance was read at 575 nm in a colorimeter. A control was simultaneously prepared taking thermally denatured enzyme. The concentration of the product (galacturonic acid) was compared to a galacturonic acid standard curve. One unit of PG activity is defined as the amount of enzyme that liberates 1 μmol of galacturonic acid per min under the assay conditions. Each data point was an average of triplicate measurements with standard deviation less than 5%. Protein was determined by Lowry method using Bovine serum albumin (BSA) as the standard (Lowry et al. 1951).
Enzyme purification
Chilled acetone was added slowly up to 30% saturation with gentle stirring at 4 °C to the crude enzyme extract. The treated crude enzyme solution was allowed to stand overnight in the refrigerator, centrifuged at 10,000 rpm for 15 min. The pellet was discarded and supernatant was saturated up to 60% with chilled acetone and allowed to stand overnight. The supernatant obtained after centrifugation at 10,000 rpm was again saturated with acetone up to 90% and was kept undisturbed at 4 °C overnight. This was centrifuged at 10,000 rpm for 15 min and the pellet obtained was dissolved in 3 mL of cold distilled water. The concentrated crude enzyme after subjecting to acetone precipitation was loaded on CM-cellulose column (6.5 × 2.0 cm) equilibrated with 10 mM citrate–phosphate buffer pH 6.0. The adsorbed protein was then washed with two times of bed volume of the same buffer. The protein was eluted stepwise using 10 mL of NaCl (0.1–2.0 M) in the citrate–phosphate (pH 6.0) buffer at the flow rate of 15 mL/h. Fractions were collected and analyzed for activity of polygalacturonase and protein. The active fractions were pooled, assayed and stored in deep fridge at −20 °C. The purity of the enzyme was checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in 10% acrylamide gel according to Laemmli (1970).
Biochemical characterization of purified polygalacturonase
The K m and k cat values of the purified enzyme were determined by measuring steady-state velocities of the enzyme catalyzed reaction at different concentrations of citrus PGA (0.05–0.6 g%) in 100 mM citrate–phosphate buffer (pH 4.0) at 40 °C and drawing double reciprocal plot. Calculations were made using linear regression analysis of the data points of the double reciprocal plot. The pH optimum was determined by measuring steady-state velocity using 0.5 g% PGA in the buffered reaction solution using different buffers at 100 mM in the pH range 1.0–12.0 and incubating the reaction mixtures at 40 °C for 30 min. The different buffers used were: hydrochloric acid–potassium chloride (pH 1.0–2.0), citrate–phosphate (pH 3.0–7.0), sodium phosphate (pH 8.0), glycine–sodium hydroxide (pH 9.0–10.0) and sodium phosphate–sodium hydroxide (pH 11.0–12.0). The pH stability of the enzyme was studied by exposing the enzyme to buffers of different pH for 24 h at 4 °C. After 24 h, the activities of the enzyme exposed to different pH were assayed and plotted in the form of relative activity versus pH at which enzyme was exposed for 24 h.
The optimum temperature for the enzyme activity was determined by assaying activity of the enzyme at different temperatures in the range 10–100 °C and plotting a graph of the relative activity versus temperature of the reaction solutions. Thermal stability of the enzyme was tested by incubating enzyme aliquots at a particular temperature (10–100 °C) for 2 h and their activities were assayed using the standard assay method.
Effect of metal ions and protein inhibitors
The effects of metal ions, such as Ca2+, Mn2+, Co2+, Cu2+, Zn2+, Hg2+, K+, Na+, Ag+ and protein inhibitors like potassium permanganate, potassium ferrocyanide and ethylene diamine tetraacetic acid (EDTA) were studied by measuring the steady-state velocity in the reaction solutions containing 1 mM of the metal ions or protein inhibitors and comparing it with the value in the absence of these ions or inhibitors.
Mode of action of purified polygalacturonase
To decide whether the purified PG is an exo- or endo-PG, a reaction solution containing 0.5 mL of 1% PGA in distilled water and 1.4 mL of 100 mM citrate–phosphate buffer (pH 4.0) was added in a test tube. The test tube was incubated in a water bath at 50 °C and was allowed to stand to maintain the temperature for 20 min, 0.5 unit of the purified enzyme was added. Three-μL aliquots of the reaction solution were withdrawn at 15, 30 and 45 min and spots of these solutions were made on thin layer chromatography (TLC) plate coated with silica gel. Spots of monogalacturonic acid as well as PGA were also made. The TLC plate was kept in a jar containing a solution of butanol, water and acetic acid in the volume ratio 5:3:2 as mobile phase. The TLC plates were air dried, sprayed with 0.2% (w/v) orcinol in methanol and 10% sulphuric acid. The plate was air dried and baked in an oven at 80 °C for 10 min. The spots were photographed.
Clarification of orange juice
The clarification of fruit juice was studied by reported method of Ishii and Yokotsuka (1972). 50 µL of purified enzyme was added to 1 mL of orange juice (pH 4.1) and the reaction was incubated at 37 °C in a water bath. The reaction was stopped by keeping the reaction mixture in boiling water for 5 min. It was centrifuged at 3000 rpm for 5 min. Percentage transmittance was measured at 660 nm with respect to controls that contained same volume of enzyme added just before keeping the reaction mixture in boiling water.
Results and discussion
Production of polygalacturonase
Solid-state fermentation was found to be most effective for polygalacturonase production (Fig. 1). Maximum production was obtained with medium M3 followed by medium M2. Tea extract was found to enhance the enzyme production. Previously we have reported pectin lyase production under solid-state fermentation from Fusarium decemcellulare where solid-state media was supplemented with tea extract (Yadav et al. 2014). Negligible polygalacturonase production was obtained under submerged fermentation. There are physiological differences between SSF and SmF, which in turn affect enzyme production in each fermentation system. SSF includes the growth and metabolism of microorganisms in the absence of or near-absence of any free flowing water. Microbial growth and product formation occur at or near the surface of the solid substrate particle. Such a system, being closer to the natural habitats of microbes, may prove more efficient in producing certain enzymes and metabolites.
Purification of polygalacturonase
The purification of polygalacturonase from Aspergillus niger MTCC 478 was performed by acetone precipitation and CM-cellulose column chromatography. The purification chart is given in Table 1.
On 5th day, the crude enzyme was harvested and the clear culture filtrate was centrifuged and subjected to acetone precipitation (60–90% saturation). The precipitate obtained after the acetone precipitation revealed 4.65-fold purification with 4.2% yield and 10.2 U/mg specific activity (Table 1). The concentrated crude enzyme after subjecting to acetone precipitation was loaded on CM-cellulose column (6.5 × 2.0 cm) equilibrated with 10 mM citrate–phosphate buffer, pH 6.0. The adsorbed protein was then washed with two times of bed volume of the same buffer. The protein was eluted stepwise using 10 mL of NaCl (0.1–2.0 M) in the citrate–phosphate (pH 6.0) buffer at the flow rate of 15 mL/h. Fractions were collected and analyzed for activity of polygalacturonase and protein. Ion-exchange chromatography resulted in 15.28-fold purification with 1.2% yield and 33.47 U/mg of specific activity (Table 1). 16-fold purification with 1.9% yield has been reported for exo-PG from Rhizopus oryzae (Yadav et al. 2012).
The purified PG was confirmed for electrophoretic homogeneity by SDS-PAGE as shown in Fig. 2. The single band corresponding to a relative molecular mass of approximately 124.0 kDa was observed (Fig. 2) which is higher than 106 kDa A. niger polygalacturonase already reported (Kant et al. 2013). Molecular weight of exo-PG from Bacillus sp. was found to be 115 kDa (Kobayashi et al. 2001).
Effect of pH on the activity and stability of PG
The optimum pH for the purified polygalacturonase was found to be 4.0 (Fig. 3a) and the enzyme was stable for a wide pH range, i.e., 3.0–11.0 (Fig. 3b) when exposed to buffers of various pH for 24 h, indicating its suitability for clarification of fruit juices. Similar pH optimum of 4.0 has been reported for an exo-PG purified from Aspergillus sojae and Paecilomyces variotii (Dogan and Tari 2008; de Lima Damasio et al. 2010). Previous reports show that majority of fungal PGs have their pH optima in acidic range (Kester and Visser 1990; Polizeli et al. 1991; Niture and Pant 2004; Yadav et al. 2012). However, pH stability range of the enzyme was wider (3.0–11.0) as compared to fungal PGs reported from A. niger (Kant et al. 2013). An endo-PG with stability in alkaline range 7.0–11.0 has recently been reported from A. fumigatus (Anand et al. 2016).
Effect of temperature on the activity and stability of PG
Optimum temperature for the purified polygalacturonase activity was found to be 50 °C (Fig. 3c) and the enzyme retained its maximum activity between 10 and 40 °C for 30 min (Fig. 3d). Similar temperature optimum has been reported for PG from R. oryzae (Yadav et al. 2012). Several fungal PGs exhibit temperature optima between 40 and 60 °C (Devi and Rao 1996; Kaur et al. 2004; Tari et al. 2008; Thakur et al. 2010). PG produced by few thermophilic fungal strains like Aspergillus sojae, Paecilomyces variotii and Thermoascus aurantiacus have temperature optima in the range of 60–70 °C (Dogan and Tari 2008; de Lima Damasio et al. 2010; Martins et al. 2007, 2013). The thermal stability of the purified enzyme is shown in (Fig. 3d) revealing a maximum stability up to 40 °C and the activity declined sharply above this temperature. Approximately 53% of the activity was retained at 50 °C.
Kinetic parameters
The apparent K m value for degradation of PGA by the purified enzyme was found to be 2.3 mg/mL. The K m value of 2.4 mg/mL has been reported for an endo-PG from A. niger (Parenicova et al. 1998). An exo-PG from A. tubingensis has K m value of 3.2 mg/mL (Kester et al. 1996). The K m values of most of the microbial PGs are in the range of 0.1–5.0 (Polizeli et al. 1991; Zhang et al. 1999; Niture et al. 2001; Singh and Rao 2002; Thakur et al. 2010a, b; Yadav et al. 2012; Martins et al. 2013; Chen et al. 2014). An exo-PG from the fungus Penicillium frequentans has very low K m value of 0.059 (Barense et al. 2001) while PG from pathogenic fungus Ustilago maydis revealed comparatively very high K m of 57.84 (Castruita-Domínguez et al. 2014). The catalytic rate constant k cat of purified PG was found to be 194 s−1 which is higher than k cat values of 90 and 70 s−1 for endo-PGs from Aspergillus japonicus and Fusarium moniliforme (Semenova et al. 2003; Niture et al. 2001). Exo-PG from alkaliphilic Bacillus had k cat value of 22.2 s−1.
Effect of metal ions and protein inhibitors
The effects of different metal ions and protein inhibitors were assessed using 1 mM concentration of each metal ion in the reaction solution (Table 2). Amongst all metal ions only Cu2+ was found to enhance the PG activity while Ag+ completely inhibited the enzyme activity. Loss of activity may be due to the destabilization of enzyme as a result of loss of surface charge–charge interaction. Enzyme inhibition by Ca2+ may be accounted to its position in Hofmeister series wherein Ca2+ and Mg2+ are more powerful hard cations and strong denaturant. Presence of these metal ions may disturb the ion pairs formed between Asp/Glu and Lys/Arg leading to the destabilization of enzyme (Zeng et al. 2014). It has been reported that activity of an acidic endo-PG from A. niger and alkaline endo-PG from A. fumigatus is enhanced by Cu2+ at 1 mM concentration (Zhou et al. 2015; Anand et al. 2016). Metal ions like Mn2+, Hg2+ and protein inhibitor KMnO4 had no effect on PG activity. EDTA and K4 (Fe)CN6 had inhibitory effect on enzyme activity.
Mode of action of purified PG
To determine the mode of action of the purified PG, TLC experiment was performed (Fig. 4). It is quite obvious that monogalacturonic acid starts appearing on TLC plates from the very beginning and clear spot is visible just after 15 min corresponding to the spot of monogalacturonic acid. This reveals exo-PG nature of the purified PG. Two exo-PGs of molecular weight 82 and 56 kDa from A. niger have been reported in the literature (Sakamoto et al. 2002).
Application of purified PG in fruit juice clarification
In general, microbial pectinases are important group of enzymes with potential applications in various industries like wine industry, paper industry, textile industry and food industry (Garg et al. 2016). Application of purified PG in clarification of fruit juice was studied on orange juice (pH 4.1) procured from the local market. The clarification of fruit juice was studied by reported method of Ishii and Yokotsuka (1972). Transmittance increased by 27% with respect to control that contained same volume of enzyme added just before keeping the reaction mixture in boiling water. Transmittance of the treated juice increased because of removal of colloidal and suspended particles in the juice especially pectin. Colloid formation due to the presence of pectin is a major challenge in fruit juice industry, which decreases the commercial value of juices. Pectin present in fruit juices are degraded by pectinases resulting in viscosity reduction and cluster formation. As a result, the juice becomes clear and more concentrated in flavor and color (Liew Abdullah et al. 2007; Kaur et al. 2004). Clarification of fruit juice can be attributed to biochemical nature of the enzyme. The purified enzyme had pH optima 4.0 and stability in the pH range 3.0–11.0. Most of the fruit juices are acidic in nature; hence an acidic polygalacturonase with stability in acidic pH range is a suitable candidate for fruit juice clarification. Several polygalacturonases from Aspergillus carbonarius and Achaetomium sp. Xz8 have been reported to have the capacities to improve the juice yield and clarity (Nakkeeran et al. 2011) and reduce the viscosity of papaya juice (Tu et al. 2015). Polygalacturonase produced by A. niger using banana peel as a substrate has been used for clarification of banana juice (Barman et al. 2015). Use of polygalacturonases from Neosartorya fisheri in clarification of apple and strawberry juice have also been reported (Pan et al. 2015). An acidic PG from A. niger has also been used for the clarification of guava juice (Kant et al. 2013). An acidic endopolygalacturonase from Penicillium oxalicum increased light transmittance of papaya pulp by 29.5% (Cheng et al. 2016).
Conclusion
An acidic exo-polygalacturonase from A. niger MTCC 478 was produced by solid-state fermentation using cost-effective media comprising of wheat bran and tea extract and was purified simply by acetone precipitation and CM-cellulose column chromatography. A relatively high molecular weight PG of 124 kDa with pH and temperature optimum of 4 and 50 °C was observed. The k cat and K m value of purified PG was found to be 194 s−1 and 2.3 mg/mL, respectively, and Cu2+ was found to enhance the PG activity. The potential of purified PG in clarification of orange juice was elucidated owing to its acidic nature.
References
Amin F, Bhatti HN, Bilal M, Asgher M (2017) improvement of activity, thermostability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int J Biol Macromol 95:974–984
Anand G, Yadav S, Yadav D (2016) Purification and characterization of polygalacturonase from Aspergillus fumigatus MTCC 2584 and elucidating its application in retting of Crotolaria juncea fiber. 3Biotech 6:201
Barense RI, Chellegatti MASC, Fonseca MJV, Said S (2001) Partial purification and characterization of exopolygalacturonase II and III of Penicillium frequentans. Br J Microbiol 32:327–330
Barman S, Sit N, Badwaik LS, Deka SC (2015) Pectinase production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J Food Sci Technol 52:3579–3589
Bird CR, Smith CJ, Ray JA, Moureau P, Bevan MW, Bird AS, Hughes S, Morris PC, Grierson D, Schuch W (1988) The tomato polygalacturonase gene and ripening- specific expression in transgenic plants. Plant Mol Biol 11(5):651–662
Castilho LR, Medronho RA, Alves TLM (2000) Production and extraction of pectinases obtained bu solid state fermentation of agroindustrial residues with Aspergillus niger. Bioresour Technol 71:45–50
Castruita-Domínguez JP, González-Hernández SE, Polaina J, Flores-Villavicencio LL, Alvarez-Vargas A, Flores-Martínez A, Ponce-Noyola P, Leal-Morales CA (2014) Analysis of a polygalacturonase gene of Ustilago maydis and characterization of the encoded enzyme. J Basic Microbiol 54(5):340–349
Chen Y, Sun D, Zhou Y, Liu L, Han W, Zeng B, Wang Z, Zang Z (2014) Cloning, expression and characterization of a novel thermophilic polygalacturonase from Caldicellulosiruptor bescii DSM 6725. Int J Mol Sci 15(4):5717–5729
Cheng Z, Chen D, Lu B, Wei Y, Xian L, Li Y, Luo Z, Huang R (2016) A novel acid-stable endo-polygalacturonase from Penicillium oxalicum CZ1028: purification, characterization and application in the beverage industry. J Microbiol Biotechnol. doi:10.4014/jmb.1511.11045
de Lima Damasio AR, da Silva TM, Maller A, Jorqe JA, Terenzi HF, Polizeli ML (2010) Purification and partial characterization of an exo-polygalacturonase from Paecilomyces variotii liquid cultures. Appl Biochem Biotechnol 160:1496–1507
Devi NA, Rao AGA (1996) Fractionation, purification and preliminary characterization of polygalacturonase produced by Aspergillus carbonarius. Enzyme Microb Tech 18:59–65
Dinu D, Nechifor MT, Stoian G, Costache M, Dinischiotu A (2007) Enzymes with new biochemical properties in the pectinolytic complex produced by Aspergillus niger MIUG 16. J Biotechnol 131:128–137
Dogan N, Tari C (2008) Characterization of three phase partitioned exo-polygalacturonase from Aspergillus sojae with unique properties. Biochem Eng J 39:43–50
Garg G, Singh A, Kaur A, Singh R, Kaur J, Mahajan R (2016) Microbial Pectinases: an ecofriendly tool of nature for industries. 3Biotech 6:47
Hadfield KA, Benett AB (1998) Polygalacturonase: many genes in search of a function. Plant Physiol 117(2):337–343
Ishii S, Yokotsuka T (1972) Clarification of fruit juice by pectin transeliminase. J Agric Food Chem 20:787–791
Jayani RS, Sk S, Gupta R (2010) screening of bacterial strains for polygalacturonase activity: its production by Bacillus sphaericus (MTCC 7542). Enzyme Res. doi:10.4061/2010/306785
Kant S, Vohra A, Gupta R (2013) Purification and physicochemical properties of polygalacturonase from Aspergillus niger MTCC 3323. Prot Exp Purif 87:11–16
Kashyap DR, Vohra PK, Chopra S, Tewari R (2001) Applications of Pectinases in commercial sector: a review. Biores Technol 77:215–227
Kaur G, Kumar S, Satyanarayana T (2004) Production, characterization and application of a thermostable polygalacturonase of a thermophilic molud Sporotrichum thermophile. Apinis Bioresour Technol 94:239–243
Kester HC, Visser J (1990) Purification and characterization of polygalacturonases produced by the hyphal fungus Aspergillus niger. Biotechnol Appl Biochem 12(2):150–160
Kester HCM, Kusters-Van Someren MA, Muller Y, Visser J (1996) Primary structure and characterization of an exopolygalacturonase from Aspergillus tubingensis. Eur J Biochem 240:238–246
Kobayashi T, Higaki N, Suzumatsu A, Sawada K, Hagihara H, Kawai S, Ito S (2001) Purification and properties of a high- molecular- weight, exo-polygalacturonase from a strain of Bacillus. Enzyme Microb Technol 29:70–75
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lara-Marquez A, Zavela-Paramo MG, Lopez-Romero E, Horacio CC (2011) Biotechnological potential of pectinolytic complexes of fungi. Biotechnol Lett 33:859–868
Liew Abdullah AG, Sulaiman NM, Aroua MK, Megat Mohd Noor MJ (2007) Response surface optimization of conditions for clarification of carambola fruit juice using a commercial enzyme. J Food Eng 81:65–71
Lowry OH, Rose Brough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Martins ES, Leite RSR, Silva R, Gomes E (2013) Purification and properties of polygalacturonase produced by thermophilic fungus Thermoascus aurantiacus CBMAI-756 on solid-state fermentation. Enzyme Res. doi:10.1155/2013/438645
Martins ES, Silva D, Leite RSR, Gomes E (2007) Production and characterization of polygalacturonase produced by thermophilic Thermoascus aurantiacus CBMAI-756 in submerged fermentation. A Van Leeuw 91:291–299
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11:266–277
Molina SMG, Pelissari FA, Vitorello CBM (2001) Screening and genetic improvement of pectinolytic fungi for degumming of textile fibres. Br J Microbiol 32:320–326
Mrudula S, Anitharaj R (2011) Pectinase production in solid-state fermentation by Aspergillus niger using orange peel as substrate. Glob J Biotechnol Biochem 6:64–71
Nakkeeran E, Umesh-Kumar S, Subramanian R (2011) Aspergillus carbonarius polygalacturonases purified by integrated membrane process and affinity precipitation for apple juice production. Biores Technol 102:3293–3297
Niture SK, Pant A (2004) Purification and biochemical characterization of polygalacturonase II produced in semi-solid medium by a strain of Fusarium moniliforme. Microbiol Res 159:305–314
Niture SK, Pant A, Kumar AR (2001) Active site characterization of the single endo-polygalacturonase produced by Fusarium moniliforme NCIM 1276. Eur J Biochem 268:832–840
Ortega LM, Kikot GE, Rojas NL, Lopez LM, Astoreca AL, Alconada TM (2014) Production, characterization, and identification using proteomic tools of a polygalacturonase from Fusarium graminearum. J Basic Microbiol 54:S170–S177
Oszmianski J, Wojdylo A, Kolniak J (2009) Effect of enzymatic mash treatment and storage on phenolic composition, antioxidant activity, and turbidity of cloudy apple juice. J Agric Food Chem 57:7078–7085
Pan X, Li K, Ma R, Shi P, Huang H, Yang P, Meng K, Yao B (2015) Biochemical characterization of three distinct polygalacturonases from Neosartorya fischeri P1. Food Chem 188:569–575
Parenicova L, Benen JA, Kester HC, Visser J (1998) pgaE encodes a fourth member of the endopolygalacturonase gene family from Aspergillus niger. Eur J Biochem 251:72–80
Pedrolli DB, Carmona EC (2009) Pectin lyase from Aspergillus giganteus: comparative study of productivity of submerged fermentation on citrus pectin and orange waste. Prikl Biokhim Mikrobiol 45:677–683
Perrone G, Mule G, Susca A, Battilani P, Pietri A, Logrieco A (2006) Ochratoxin A production and amplified fragment length polymorphism analysis of Aspergillus carbonarius, Aspergillus tubingensis, and Aspergillus niger strains isolated from grapes in Italy. Appl Environ Microbiol 72:680–685
Polizeli MLTM, Jorge JA, Terenzi HF (1991) Pectinase production by Neurospora crassa: purification and biochemical characterization of extracellular polygalacturonase activity. J Gen Microbiol 137:1815–1823
Sakamoto T, Bonnin E, Quemener B, Thibault JF (2002) Purification and characterization of two exo-polygalacturonases from Aspergillus niger able to degrade xylogalacturonan and acetylated homogalacturonan. Biochim Biophys Acta 1572(1):10–18
Semenova MV, Grishutin SG, Gusakov AV, Okunev ON, Sinitsin AP (2003) Isolation and properties of Pectinases from the fungus Aspergillus japonicas. Biochem (Moscow) 68:559–569
Silva D, Martins ES, Silva R, Gomes E (2002) Pectinase production by Penicillium viridicatum RFC3 by solid state fermentation using agricultural wastes and agro-industrial byproducts. Br J Microbiol 33:318–324
Singh SA, Rao AGA (2002) A simple fractionation protocol for, and a comprehensive study of the molecular properties of, two major endopolygalacturonases from Aspergillus niger. Biotechnol Appl Biochem 35(2):115–123
Takao M, Nakaniwa T, Yoshikawa K, Terashita T, Sakai T (2000) Purification and characterization of thermostable pectate lyase with protopectinase activity from thermophilic Bacillus sp. TS 47. Biosci Biotechnol Biochem 64:2360–2367
Tari C, Dogan N, Gogus S (2008) Biochemical and thermal characterization of crude exo- polygalacturonase from Aspergillus sojae. Food Chem 111:824–829
Tariq A, Latif Z (2012) Isolation and biochemical characterization of bacterial isolates producing different levels of polygalacturonase from various sources. Afr J Microbiol Res 6(45):7259–7264
Thakur A, Pahwa R, Singh S, Gupta R (2010) Production, purification, and characterization of polygalacturonase from Mucor circinelloides ITCC 6025. Enzyme Res. doi:10.4061/2010/170549
Tu T, Luo H, Meng K, Cheng Y, Ma R, Shi P, Huang H, Bai Y, Wang Y, Zhang L, Yao B (2015) Improvement in thermostability of an Achaetomium sp. Strain Xz8 endopolygalacturonase via the optimization of charge–charge interactions. Appl Environ Micrbiol 81(19):6938–6944
Yadav S, Anand G, Dubey AK, Yadav D (2012) Purification and characterization of an exo-polygalacturonase secreted by Rhizopus oryzae MTCC1987 and its role in retting of Crotolaria juncea fibre. Biologia 67(6):1069–1074
Yadav S, Dubey AK, Anand G, Kumar R, Yadav D (2014) Purification and biochemical characterization of an alkaline pectin lyase from Fusarium decemcellulare MTCC 2079 suitable for Crotolaria juncea fiber retting. J Basic Microbiol 54:S161–S169
Yadav S, Shastri NV (2007) Purification and properties of an extracellular pectin lyase produced by the strain of Penicillium oxalicum in solid-state fermentation. Indian J Biochem Biophys 44:247–251
Yadav PK, Singh VK, Yadav S, Yadav KDS, Yadav D (2009) In silico analysis of pectin lyase and pectinase sequences. Biochem (Moscow) 74(9):1049–1055
Zeng J, Gao X, Dal Z, Tang B, Tang X-F (2014) Effects of metal ions on stability and activity of hyperthermophilic pyrrolysine and further stabilization of this enzyme by modification of a Ca2+-binding site. Appl Environ Microbiol 80:2763–2772
Zhang J, Bruton BD, Biles CL (1999) Fusarium solani endo-polygalacturonase from decayed muskmelon fruit: purification and characterization. Physiol Mol Plant Pathol 54:171–186
Acknowledgements
GA would like to acknowledge Jawaharlal Nehru Memorial Fund for providing Jawaharlal Nehru Memorial Scholarship for doctoral studies. The financial support by Department of Science and Technology, Government of India, New Delhi, in the form of SERB Young Scientist Fellowship (SB/FT/LS-430/2012) to S. Yadav is also thankfully acknowledged. The authors acknowledge the infrastructural support from the Head, Department of Biotechnology, D.D.U. Gorakhpur University, Gorakhpur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anand, G., Yadav, S. & Yadav, D. Production, purification and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotech 7, 122 (2017). https://doi.org/10.1007/s13205-017-0760-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0760-3