Abstract
The paper presents the kinetics of Zn (II) ions adsorption as well as its dependence as a function of pH on nanostructured zirconium phosphate and its composites with silica and titania. The nanostructured zirconium phosphate-containing composites were obtained by mechanical processing in the Pulverisette-7 (Fritsch Gmbh) mill. The obtained composites were characterized by a heterogeneous surface coverage of silica gel or titanium oxides. Zinc ions adsorption studies on these adsorbents showed practically complete removal of Zn (II) from aqueous solutions with an initial concentration of < 0.0001 mol/dm3 and a pH > 4 within 10 min.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of civilization related to the consumption of modern products causes an increased demand for heavy metals and their compounds. Their production and processing are made in various industries which in the technological processes related to production generate wastes containing heavy metals. These wastes include sewages treated before they are discharged into the environment. To treat wastewaters containing heavy metals, chemical precipitation in the form of insoluble compounds combined with sedimentation or filtration, ion exchange, electrodialysis, flotation, reverse osmosis, ultrafiltration or adsorption are used (Fua and Wang 2011; Renu et al. 2017; de Dias et al. 2019; Altunay et al. 2020; Aboobakri and Jahani 2020; Chen and Wang 2004; Shirsath and Shirivastava 2015; Kaur 2016). Another application of the adsorption methods on new composite materials is the preconcentration of metals species in the chemical analysis (Kaur 2016) or the preparation of new catalysts (Chiang et al. 2019). Adsorption on new composite materials can also be used to remove other troublesome pollutants in sewage and water, such as dyes (Wiśniewska et al. 2018).
. The adsorption of heavy metal ions is a cheap and effective method of removing metal ions from wastewaters and waters contaminated with these metals consisting in using various adsorbents of natural and synthetic origins (Lim et al. 2013; Falayi et al. 2014; Tan et al. 2018; Theron et al. 2008).
Zinc is a micronutrient, the presence of which is essential for the proper functioning of the human body but its overdose may lead to its poisoning. A zinc overdose is especially dangerous for animals (Kabata et al. 1999). Due to overdosing, zinc ions are among those whose concentration is controlled in the environment. Zinc is an element commonly found in the earth's crust but its significant amounts are emitted from metallurgical and processing plants. Moreover, it is used in alloys, anti-corrosion coatings, in the paint, pharmaceutical and cosmetic industries. Soluble zinc salts get into the water and hence it can be absorbed and bioaccumulated by living organisms (Altunay et al. 2020; Eisler 1993).
Studies of the adsorption of zinc ions on the interface metal oxide (SiO2, Fe2O3 and TiO2)/electrolyte solution as a function of pH showed that in the narrow pH range, cation adsorption increases rapidly creating an adsorption edge (Janusz et al. 2000, 2003; Chibowski et al. 2002). The adsorption edge is characterized by the pH50% parameter, i.e. the pH value at which 50% of the total amount of cations is adsorbed. Besides, the ΔpH10–90% parameter is related to the pH range in which the cation adsorption increases from 10 to 90% and characterized by the slope of the adsorption edge (Robertson et al. 1997). For the above-mentioned oxides, the pH50% of the adsorption of zinc ions from the solutions with an initial concentration of 0.000001 mol/dm3 which characterizes the affinity of ions for the surface, was: 6.2 for SiO2 (Falayi et al. 2014). 6.5 for α-Fe2O3 (Lim et al. 2013) and 5.1 for TiO2 (Chen and Wang 2004). The tested adsorbents can effectively remove zinc ions from neutral or alkaline solutions.
Due to the higher Bronsted acidity, zirconium phosphate ZrP is promising as an adsorbent of cations (Naushad 2009; Clearfield 1982). However, amorphous ZrP is characterized by a high specific surface area but low accessible due to a sufficiently large content of micropores which impairs its kinetic characteristics and reduces the efficiency of its application in the cation-exchanging processes. The creation of composite materials is one of the ways to overcome this disadvantage. Particularly, the deposition onto the supports with a large specific surface area and developed mesoporosity is one of the most effective methods to improve ZrP properties (Zhang et al. 2008a, b; Perlova et al. 2017). It should be noted that mechanochemical method was previously used for the improvement of adsorption properties both of bulk and supported phosphorus-containing adsorbents (Janusz et al. 2010; Zakutevskyy et al. 2020).
This paper presents the studies of the kinetics and statics of zinc ions adsorption on zirconium phosphate (ZrP) and its composites with SiO2 or TiO2, the synthesis and properties of which were described in our previous papers (Khalameida et al. 2017; Sydorchuk et al. 2012).
Experimental
Reagents
Fumed silica (aerosils A-50 and A-380) and titania (Oriana, Ukraine) with the specific surface area 45, 346 and 64 m2 g−1, respectively, were utilized as supports for the preparation of deposited samples. Aerosils are amorphous and titania consists of anatase and rutile phases.
Synthesis procedures
The procedures of ZrP deposition (20%w/w) were as follows:
Appropriate amounts of reagents, namely ZrP dried xerogel or hydrogel with 88% w/w humidity and fumed titania or aerosils A-50 (A-380), were subjected to mechanochemical treatment (MChT) (milling) at 300 rpm for 0.5 h via Pulverisette-7 (Fritsch Gmbh). The milling was carried out on air (dry milling) or in water (wet milling). Water was not added into the mill when ZrP hydrogel was used. After the wet milling, the dispersions were dried at 20 °C for 72 h. The bulk ZrP, used during milling was prepared through precipitation by H3PO4 from aqueous solutions of ZrOCl2·8H2O as in (Janusz et al. 2000).
Study of physical–chemical characteristics
The starting reagents and products of their mechanochemical transformations were studied by means of X-ray powder diffraction (XRD) using a diffractometer Philips PW 1830 with CuKα- radiation. The FTIR spectra in the range 4000–1400 cm−1 were registered using the spectrometer “Spectrum-One” (Perkin-Elmer). The ratio of the sample and KBr powders was 1:20. KBr was dried at 600 °C for 2 h before the measurements.
SEM and EDS analysis
The zirconium phosphate samples and the ZrP/SiO2 and ZrP/TiO2 composites were covered with a conductive carbon coater, mounted in the specimen holder and transferred to the Quanta 3D FEG scanning electron microscope, FEI Company. The qualitative and quantitative surface analyses of the main elements of zirconium phosphate samples were conducted from the EDS spectra collected using the FEI Quanta 3D FEG scanning electron microscope equipped with the EDS spectrometer.
Analysis of porous structure
The parameters of porous structure (specific surface area S, sorption pore volume Vs, micropores volume Vmi, mesopores volume Vme) were determined from the isotherms of low-temperature nitrogen adsorption obtained by means of the analyzer ASAP 2405 N (“Micromeritics Instrument Corp”). The outgassing temperature and duration were 150 °C and 2 h, respectively. Meantime the value of Vs was determined at the relative nitrogen pressure close to 1, the values of Vmi and Vme were calculated using the t-method and the BJH-method, respectively. The total pore volume VΣ was determined via impregnation of the samples by liquid water, previously dried at 150 °C. The following relations are valid between these parameters:
whereVma—the volume of macropores which are not filled in the process of adsorption from the vapour phase but filled by impregnation from the liquid phase.
The mesopore diameter dPSD was calculated from the curves of the pore size distribution (PSD) using the desorption branch of isotherms according to the BJH-method.
Zn (II) adsorption measurements
The adsorption densities of Zn (II) ions were measured using the radiotracer technique (Zn-65 radioisotope) was submitted by OPiDI (Świerk, Poland). Based on the radioactivity changes before and after the sorption of Zn (II) ions on the surface of zirconium phosphate samples, adsorption was calculated. The details of the method are described in the literature (Janusz and Skwarek 2018). To compare the adsorption affinity, the ZrP, ZrP/SiO2 or ZrP/TiO2 samples were added to 50 ml of Zn (II) ions solution so that the total surface area of the sample to volume ratio of the solution was the same. Radioactivity of the electrolyte solution before and after adsorption was measured using the liquid scintillation counter by Beckman, USA.
Results and discussion
Physicochemical characteristics
The XRD analysis shows that ZrP bulk precipitated in acid medium is X-ray amorphous (Kabata et al. 1999; Janusz et al. 2000; Zhang et al. 2008a, b). Its mechanochemical deposition onto the fumed oxides does not phase the composition of prepared compositions. Thus, the samples based on aerosils retain being X-ray amorphous since both components are the same. On the other hand, the XRD patterns for the TiO2 samples contain peaks of anatase and rutile as for the support itself. Since the obtained diffraction patterns are not very informative, they are not shown here.
Figure 1a and b show SEM micrographs of samples of zirconium phosphate composites with A380 and A50 aerogels, respectively. SEM micrographs indicate aggregation of large and small particles in composites obtained as a result of mechanochemical treatment of samples. In the case of the ZrP/A380 composite, there is a visible presence of large non-uniform particles in size (~ 400 nm) and shape, and small almost spherical particles, more homogeneous in size (~ 36 nm). The sample of the ZrP/A50 composite, Fig. 1b, obtained as a result of the mechanochemical treatment, also indicates the heterogeneity of the size and shape. However, large particles with a size of ~ 700 nm show a distinct character of small particle agglomerates with a spherical shape and similar size, ~ 65 nm.
The morphology of the particles of zirconium phosphate/titanium oxide composites obtained as a result of mechanochemical treatment is shown in Fig. 2a–c for the samples: 20% Zr(HPO4)2/TiO2 MChT of Zr(HPO4)2 xerogel in air, 20% Zr(HPO4)2/TiO2 MChT of Zr(HPO4)2 xerogel in the water at 300 rpm during 0.5 h and samples 20% Zr(HPO4)2/TiO2 MChT of Zr(HPO4)2 MChT hydrogel in the water at 300 rpm during 0.5 h, respectively. Each of the presented Figures shows a fraction of larger spherical particles with a diameter of ~ 130 nm and a fraction of spherical particles with a smaller diameter ~ 57 nm in the case of the treatment of xerogel samples in air or particles with a diameter of ~ 75 nm of composites mechanically processed in water.
The results of the analysis of the surface composition of ZrP/SiO2 and ZrP/TiO2 samples by the EDS method are presented in Table 1. As can be seen, the content of ZrP on the surface of the composites reaches a few percent. This means that only a part of the surface of the SiO2 or TiO2 support is covered with ZrP. Moreover, the analysis of the data presented in Table 1 shows that the ZrP/SiO2 composite milled in the air (sample 2) is characterized by the lowest content of ZrP on the ZrP/SiO2 surface. The highest surface ZrP content among ZrP/SiO2 composites was obtained by the method of mechanical treatment of the mixture containing ZrP in the form of a wet gel (sample 6).
In the case of ZrP/fumed TiO2 composites, mechanical treatment in the air also leads to the smallest surface content of ZrP, although the difference between the samples, mechanically treated in water and in the air (samples 4 and 3, respectively), is smaller. This difference can be explained as follows. Taking into account a large excess of silica in relation to ZrP, as well as the difference in the hardness of the components (Mohs hardness of amorphous silica and ZrP is 5 and 6.5, respectively) (Wood and Hodgkiess 1972; Davraz and Gunduz 2005), "smearing" of amorphous silica on the ZrP particles is possible during dry milling. To a lesser extent, this applies to the composition based on crystalline TiO2. If the phase, that is deposited (ZrP), is in the form of a wet gel (samples 6 and 7), the Zr/Si(Ti) ratio is 1.5–2 times higher than during deposition of a dried ZrP xerogel (samples 2–4). Similar results were obtained during the mechanochemical deposition of tin dioxide on silica gel (Khalameida et al. 2020).
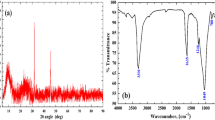
The FTIR spectra of the studied samples for the range 4000–1400 cm−1, presented in Figs. 3 and 4, confirm the results of EDS analysis. As can be seen, these spectra contain broad absorption bands (a.b.) at 3800–2700 cm−1 which consist of several components and are attributed to the stretching vibrations of the surface OH-groups. Particularly, the spectra recorded for samples based on aerosils contain sharp a.b. at 3745 cm−1 and broad a.b. with the maximum about 3300 cm−1. The former is assigned to the isolated silanol groups (Innocenzi 2003; Skubiszewska-Zieba et al. 2016; Christy 2011) and the latter—to the P–OH groups Zhang et al. 2008a, b; Pica 2017; Rao et al. 2017; Jastrzębski et al. 2011). Similarly, the spectra for samples based on TiO2 contain a.b. at 3550 cm−1 and 3300 cm−1. The first of these bands is attributed to the stretching vibrations of Ti–OH groups (Deiana et al. 2010; Cerrato et al. 1993). Therefore, the spectroscopic data show that the deposited ZrP phase does not cover completely the surface of the support and the surface of the compositions has a mosaic structure.
Bulk ZrP has a micro-mesoporous structure (Sydorchuk et al. 2012). Its mechanochemical deposition onto fumed oxides in water facilitates meso-macroporous compositions formation. As a result, the isotherms characteristic for mesoporous materials were recorded for these supported samples (Rouquerol et al. 1994). Besides, PSD curves have several maxima in the range of mesoporosity [inset to Fig. 1 in Sydorchuk et al. (2012)]. The parameters of their porous structure are listed in Table 2. One can see that sample 2 prepared by dry milling possesses a maximal specific surface area. This milled sample is a highly dispersed and non-porous powder, as found by dry milling of pure aerosil in work (Sydorchuk et al. 2010). The other studied samples have developed mesoporous or meso-macroporous structure i.e. they are characterized by the multimodal porosity which accelerates diffusion in porous space. The excess of the VΣ value over the Vs one confirms the presence of macropores. As a result, porous structure of deposited compositions and cation-exchanged sites should be more accessible for hydrated Zn species.
Kinetics of Zn (II) adsorption on ZrP
The kinetics of zinc ions adsorption on the ZrP/SiO2 composite samples from a solution with an initial concentration of Zn (II) ions of 0.000001 mol/dm3 and an initial concentration of 0.0001 mol/dm3 are presented, in Figs. 5 and 6, respectively. Obviously, the faster kinetics for the supported samples compared with bulk ZrP is due to their multimodal porosity: the absence of micropores but the presence of macropores and mesopores of different sizes (Table 2). The latter facilitates fast diffusion of hydrated cations in the pores and accessibility of surface cation-exchanged sites. The pseudo-first-order, pseudo-second-order, intra-particle diffusion and Elovic models were fitted to the time dependence of adsorption, as in works (Tan et al. 2018; Zhang et al. 2008a, b; Shafaati et al. 2020; He et al. 2020; Bulin et al. 2020). The best fit of the experimental results was obtained using the pseudo-second-order model as for sorption of Cd(II) on modified attapulgite (He et al. 2020) or Cr (VI) using reduced graphene oxide/montmorillonite composite (Peng et al. 2020). The comparison of the experimental data (points) to the model calculations with the second-order model (lines) is shown in Figs. 5 and 6 (points) and the constants of the fit with the pseudo-second-order model are presented in Tables 3 and 4 for the initial concentrations of 0.000001 and 0.0001 mol/dm3. As can be seen from the data of Tables 3 and 4, the equilibrium adsorption for all samples is the same except the sample No. 1 (ZrP), namely for the Zn (II) ions adsorption from solution with the initial concentration of 0.000001 mol/dm3. However, in this case, the adsorption pH, due to the acidic nature of the zirconium phosphate surface groups, was much lower than for the other samples (pH 3 against pH ~ 6, respectively). Since the adsorption of Zn (II) ions takes place through the exchange of hydrogen ions of surface groups, a lower pH value reduces the adsorption of Zn (II) ions. The presented results of the kinetics of Zn (II) adsorption show that the adsorption of zinc ions on both bulk zirconium phosphate and its composites with SiO2 reaches equilibrium within a few minutes.
Kinetics Zn (II) ions adsorption of on the zirconium phosphate/silica (sample no 2 and 6) and zirconium phosphate/titania (sample no4) composites samples from the solution of the initial concentration 0.000001 mol/dm3 Zn (II) ions. The points indicate the experimental data, the lines-the pseudo-second-order model using the constants collected in Table 3
Kinetics Zn (II) ions adsorption of Zn (II) ions on the zirconium phosphate/silica or titania composites samples from the solution of the initial concentration 0.0001 mol/dm3 Zn (II) ions. The points indicate the experimental data, the lines-the pseudo-second-order model using the constants collected in Table 4
The dependence of Zn (II) ions adsorption at the ZrP interface as a function of pH for different concentrations of Zn (II) ions is shown in Fig. 7 while for the ZrP/SiO2 and ZrP/TiO2 composites it is presented for the initial concentration of 0.000001 mol/dm3 Zn (II) ions in Fig. 8, and for the initial concentration of 0.0001 mol/dm3 in Fig. 9. The course of adsorption of Zn (II) ions at the above-mentioned phase boundaries as a function of pH resembles the adsorption of cations at the interface metal oxide—electrolyte solution interface, which is characterized by the edge adsorption, i.e. a narrow pH range in which the adsorption increases from almost 0–100%. The characteristic parameters of the edge are pH50% adsorption and ΔpH10–90%. The first parameter indicates the adsorption affinity of ions for the surface of a solid, the latter determines the slope of the adsorption edge.
The adsorption of cations on zirconium phosphate, similarly to the metal oxide, can be described as a process of non-stoichiometric cation exchange with a hydrogen atom from a surface hydroxyl group on a metal oxide (Bulin et al. 2020).
Where≡SOH denotes the surface group ZrP (≡ZrOH or = POH).M—denotes the metal cation (Zn2+).n—the number of surface groups reacting with the cation = the number of released H + cations.z—the electric charge of the cation (2 +).
Assuming that the energy of the chemical interactions of the ion with the surface group is much greater than that of the electrostatic interactions, the thermodynamic constant of the cation adsorption reaction on the zirconium phosphate surface, omitting the electrostatic interaction component, can be expressed by the following formula:
where
βns—the thermodynamic constant of the reaction.
When the equation is logged and transformed, a linear form is obtained that allows to calculate the equilibrium constant of the reaction βns and the n factor related to the amount of released ions H+:
The values of the constants and the amount of released H+ ions obtained by this method change with the concentration of the adsorbed ion and that of the carrier electrolyte. However, a good match of the adsorption as a function of pH is achieved for a given concentration of the ion and the background electrolyte.
The relations presented in Figs. 7–9 show only a part of the adsorption edge. In the case of zirconium phosphate, the adsorption edge is shifted towards the acidic pH scale, the pH50% calculated using the non-stoichiometric exchange model is 1.2, while the number of H+ ions exchanged is 0.9. The pH50% value indicates a very high affinity of Zn (II) ions for the ZrP surface at the initial concentration 0.000001 mol/dm3 Zn (II): in the case of TiO2 (anatase) pH50% ~ 5.5 (Janusz et al. 2000, 2003) and for SiO2 pH50% = 6.2 (Janusz et al. 2003).
The computations of the equilibrium constants of Zn (II) adsorption and the amount of released H+ ions based on the non-stoichiometric exchange model are presented in Table 5, taking into account the following concentrations of the surface hydroxyl groups: at ZrP surface 4.2 OH groups/nm2 (Tang et al. 2016), at silica 4.9 OH groups/nm2 (Zhuravlev 1987) and TiO2 4.8 OH groups/nm2 (Wu et al. 2017). In most cases of Zn (II) adsorption on zirconium phosphate and composite materials, the amount released by one Zn (II) ion is approximately equal to 1 H+ ion. This is an approximate value because the slope of the line representing the relationship lg {[(≡SO-)nZn2−n]/[Zn2+]} vs. lg{[H+]/[≡SOH]} apart from the value of n also contains a component of electrostatic interactions. Nevertheless, reaction 1 represents a certain result of the adsorption reaction on one or two functional groups and allows to determine the participation of these reactions in the adsorption of Zn (II) ions and surface groups. In the case of ZrP/SiO2 composites, pH50% = 3.4 and for ZrP/TiO2 composites, pH50% = 3.1. This may be due to the contribution of weaker acidic Si–OH and Ti–OH groups (see FTIR data in Figs. 1 and 2). An increase in the initial concentration of Zn (II) ions causes a shift of the adsorption edge towards higher pH values and its flattening (Table 5).
Conclusions
The nanostructured compositions containing 20% zirconium phosphate and 80% silicon dioxide or titanium dioxide prepared through milling the mixture of precipitated zirconium phosphate with fumed oxides at 300 rpm for 0.5 h contain only a few % of zirconium phosphate on the surface and have developed meso- and meso-macroporous structure if milling is carried out in the presence of water. Strong P-OH as well as weak Si–OH and Ti–OH groups are present on their surface. The adsorption of Zn (II) ions on nanostructured zirconium phosphate and its composites with oxides reaches equilibrium within 10 min and is well described by the pseudo-second-order kinetic equation.
Location of the Zn (II) adsorption edge on zirconium phosphate from the solutions with an initial concentration of 0.000001 mol/dm3, pH50% falls at pH = 1.3, while for a concentration of 0.001 mol/dm3 pH50% = 3.2. These values indicate that bulk zirconium phosphate can remove Zn (II) ions from the aqueous solutions in the acidic environment effectively. Research on the adsorption of Zn (II) ions on the ZrP/SiO2 and ZrP/TiO2 composites indicates that these composites can remove Zn (II) ions from aqueous solutions with pH > 5 effectively.
References
Aboobakri E, Jahani M (2020) Graphene oxide/Fe3O4/polyaniline nanocomposite as an efficient adsorbent for the extraction and preconcentration of ultra-trace levels of cadmium in rice and tea samples. Res Chem Intermed 46(4):2347–2374
Altunay N, Tuzen M, Hazer B, Elik A (2020) Usage of the newly synthesized poly(3-hydroxy butyrate)-b-poly(vinyl benzyl xanthate) block copolymer for vortex-assisted solid-phase microextraction of cobalt (II) and nickel (II) in canned foodstuffs. Food Chem 321:126690
Bulin C, Li B, Zhang Y, Zhang B (2020) Removal performance and mechanism of Fe3O4/graphene oxide as an efficient and recyclable adsorbent toward aqueous Hg(II). Res Chem Intermed 46(3):1–12
Cerrato G, Marchese L, Morterra C (1993) Structural and morphological modifications of sintering microcrystalline TiO2: an XRD, HRTEM and FTIR study. Appl Surf Sci 70:200–205
Chen JP, Wang L (2004) Characterization of metal adsorption kinetic properties in batch and fixed-bed reactors. Chemosphere 54:397–404
Chiang Hsieh LH, Ou HH, Huang ChW (2019) Adsorption of Cu(II) in aqueous solution using microwave-assisted titanate nanotubes. Appl Nanosci 9:505–514
Chibowski S, Janusz W (2002) Specific adsorption of Zn(II) and Cd(II) ions at the α-Fe2O3/electrolyte interface-structure of the electrical double layer. Appl Surf Sci 196:343–355
Christy A (2011) Effect of hydrothermal treatment on adsorption properties of silica gel. Ind Eng Chem Res 50:5543–5549
Clearfield A (1982) Inorganic ion exchanger materials. CRC Press, Boca Raton
Davraz M, Gunduz L (2005) Engineering properties of amorphous silica as a new natural pozzolan for use in concrete. Cement Concrete Res 35:1251–1261
de Dias FS, Guarino MEPA, Costa Pereira AL, Pedra PP, de Bezerra MA, Marchetti SG (2019) Multivariate optimization of ultrasound-assisted liquid–liquid microextraction based on two solvents for cadmium preconcentration prior to determination by flame atomic absorption spectrometry. Microchem J 146:1095–1110
Deiana C, Fois E, Coluccia S, Martra G (2010) Surface structure of TiO2 P25 nanoparticles: infrared study of hydroxy groups on coordinative defect sites. J Phys Chem C 114:21531–21538
Eisler R, (1993) Zinc Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review. Biological Report 10 Contaminant Hazard Reviews, Report 26, U.S. Department of the Interior Fish and Wildlife Service, Patuxent Wildlife Research Center, Laurel, Maryland 20708
Falayi T, Ntuli F (2014) Removal of heavy metals and neutralisation of acid mine drainage with un-activated attapulgite. J Ind Eng Chem 20:1285–1300
Fua F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
He Y, Sun X, Zhang P, Wang F, Zhao Z, He C (2020) Cd(II) adsorption from aqueous solutions using modified attapulgite. Res Chem Intermed 46(2):1–12
Innocenzi P (2003) Infrared spectroscopy of sol-gel derived silica-based films: a spectra-microstructure overview. J Non-Cryst Solids 316:309–319
Janusz W, Skwarek E (2018) Effect of Co(II) ions adsorption in the hydroxyapatite/aqueous NaClO4 solution system on particles electrokinetics. Physicochem Probl Min 54:31–39
Janusz W, Jabłoński J, Sprycha R (2000) The Electrical interfacial layer at the TiO2 (anatase)/electrolyte interface – adsorption of Zn(II) and Cd(II) ions. J Dispers Sci Technol 21:739–759
Janusz W, Patkowski J, Chibowski S (2003) Competitive adsorption of Ca2+ and Zn (II) ions at monodispersed SiO2/electrolyte solution interface. J Colloid Interface Sci 266:259–268
Janusz W, Khalameida S, Sydorchuk V, Skwarek E, Zazhigalov V, Skubiszewska-Zięba J, Leboda R (2010) Some properties of milled vanadium phosphates. Adsorption 16(4–5):333–341
Jastrzębski W, Sitarz M, Rokita M, Bułat K (2011) Infrared spectroscopy of different phosphates structures. Spectrochimica Acta Part A Mol Biomol Spect 79(4):722–727
Kabata-Pendias A, Pendias H, 1999, Biogeochemia pierwiastków śladowych, PWN, 344.
Kaur A (2016) Applications of organo-silica nanocomposites for SPNE of Hg(II). Appl Nanosci 6:183–190
Khalameida S, Sydorchuk V, Skubiszewska-Zięba J, Charmas B, Skwarek E, Janusz W (2017) Hydrothermal, microwave and mechanochemical modification of amorphous zirconium phosphate structure. J Therm Anal Calorim 128:795–806
Khalameida S, Sydorchuk V, Levytska S, Shcherban N (2020) Physicochemical and photocatalytic properties of tin dioxide supported onto silica gel. J Therm Anal Calorim 140:2131–2142
Lim AP, Aris AZ (2013) A review on economically adsorbents on heavy metals removal in water and wastewater. Rev Environ Sci Biotechnol 13:163–181
Naushad M (2009) Inorganic and composite ion exchange materials and their applications. Ion Exch Lett 2:1–14
Peng C, He Z, Feng J, Chen D, Ding H, Wang J, Du G (2020) Preparation of reduced graphene oxide/montmorillonite composite hydrogel. Res Chem Intermed 46(12):1–15
Perlova N, Dzyazko Y, Perlova O, Palchik A, Sazonova V (2017) Formation of zirconium hydrophosphate nanoparticles and their effect on sorption of uranyl cations. Nanosc Res Let 12(1):209
Pica M (2017) Zirconium phosphate catalysts in the XXI century: state of the art from 2010 to date. Catalysts 7(6):190
Rao KTV, Souzanchi S, Yuan Z, Ray MB, Xu C (2017) Simple and green route for preparation of tin phosphate catalysts by solid-state grinding for dehydration of glucose to 5-hydroxymethylfurfural (HMF). RSC Adv 7(76):48501–48511
Renu B, Agarwal M, Singh K (2017) Methodologies for removal of heavy metal ions from wastewater: an overview. Interdis Environ Rev 18(2):124–142
Robertson AP, Leckie JO (1997) Cation binding predictions of surface complexation models: effects of pH, Ionic strength, cation loading, surface complex, and model fit. J Colloid Interface Sci 188:444–456
Rouquerol J, Avnir D, Fairbridge CW, Everett DH, Haynes JM, Pernicone N, Ramsay JDF, Sing KSW, Unger KK (1994) Recommendations for the characterization of porous solids. Pure Appl Chem 66(8):1739–1758
Shafaati M, Miralinaghi M, Shirazi RHSM, Moniri E (2020) The use of chitosan/Fe3O4 grafted graphene oxide for effective adsorption of rifampicin from water samples. Res Chem Intermed 46(4):2247–2274
Shirsath DS, Shirivastava VS (2015) Adsorptive removal of heavy metals by magnetic nanoadsorbent: an equilibrium and thermodynamic study. Appl Nanosci 5:927–935
Skubiszewska-Zieba J, Khalameida S, Sydorchuk V (2016) Comparison of surface properties of silica xero- and hydrogelshydrothermally modified using mechanochemical, microwave and classical methods. Colloids Surf A Physicochem Eng Aspects 504:139–153
Sydorchuk V, Khalameida S, Zazhigalov V, Skubiszewska-Zięba J, Leboda R, Wieczorek-Ciurowa K (2010) Influence of mechanochemical activation in various media on structure of porous and non-porous silicas. Appl Surf Sci 257:446–450
Sydorchuk V, Janusz W, Khalameida S, Skwarek E, Skubiszewska-Zieba J, Leboda R, Zazhigalov V (2012) Synthesis, structure and some properties of zirconium phosphate/ oxide support compositions. Therm Anal Calorim 108:1009–1016
Tan B, Zhao H, Zhang Y, Quan X, He Z, Zheng W, Shi B (2018) Amphiphilic PA-induced three-dimensional graphene macrostructure with enhanced removal of heavy metal ions. J Colloid Interface Sci 512:853–861
Tang M, Yang TS, Zhang Y (2016) A brief review on α-zirconium phosphate intercalation compounds and nano-composites. Sci China Techn Sci 59:436–441
Theron J, Walker JA, Cloete TE (2008) Nanotechnology and water treatment: applications and emerging opportunities. Crit Rev Microbiol 34(1):43–69
Wiśniewska M, Wawrzkiewicz M, Polska-Adach E, Fijałkowska G, Goncharuk O (2018) Nanosized silica–titanium oxide as a potential adsorbent for C.I. Acid Yellow 219 dye removal from textile baths and wastewaters. Appl Nanosci 8:867–876
Wood GC, Hodgkiess T (1972) The hardness of oxides at ambient temperatures. Mater Corros 23:766–773
Wu ChY, Tu KJ, Deng JP, Lo YS, Wu CH (2017) Markedly enhanced surface hydroxyl groups of TiO2 nanoparticles with superior water-dispersibility for photocatalysis. Materials 10:566–579
Zakutevskyy OI, Sydorchuk VV, Khalameida SV, Kovtun MF (2020) Sorption properties of bulk and deposited ammonium molibdophosphate with respect to Cs+, Sr2+, and UO22+ ions. Theor Exp Chem 56(3):205–211
Zhang C, Lin C, Li C, Quan Z, Liu X, Lin J (2008a) Enhanced Luminescence of BPO4 by Mixing with SiO2 and Al2O3. J Phys Chem C 112:2183–2219
Zhang QR, Du W, Pan BC, Pan BJ, Zhang WM, Zhang QJ, Xu ZW, Zhang QX (2008b) A comparative study on Pb2+, Zn2+ and Cd2+ sorption onto zirconium phosphate supported by a cation exchanger. J Hazard Mater 152:469–475
Zhuravlev LT (1987) Concentration of hydroxyl groups on the surface of amorphous silicas. Langmuir 3:316–318
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, I state that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janusz, W., Sydorchuk, V., Skwarek, E. et al. Adsorption affinity of Zn (II) ions for nanostructured zirconium phosphate/silica or titania composites. Appl Nanosci 12, 725–734 (2022). https://doi.org/10.1007/s13204-021-01722-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-01722-w