Abstract
Gum kondagogu reduced/stabilized silver nanoparticles (GK-AgNPs) were evaluated for their increased antibacterial and antibiofilm activities in combination with various antibiotics (ciprofloxacin, streptomycin and gentamicin) against Gram-positive (Staphylococcus aureus 25923, Staphylococcus aureus 49834) and Gram-negative (Escherichia coli 25922, Pseudomonas aeruginosa 27853) bacteria. The micro-broth dilution assay suggested an enhanced antibacterial activity of GK-AgNPs in combination with ciprofloxacin and aminoglycosides (streptomycin and gentamicin) against tested strains. Though the antibacterial activity of GK-AgNPs was found to increase significantly in the presence of antibiotics, the % enhancement was found to depend on both types of antibiotic and bacterial strain. It was also found that GK-AgNPs (1 µg/mL) in combination with various antibiotics at sub-MIC concentrations could inhibit 70 % of the bacterial biofilm formation as compared to respective controls. The enhanced antibacterial activity was due to the increased production of intracellular reactive oxygen species in bacteria when treated with a combination of GK-AgNPs and streptomycin as compared to individual treatment. The increased oxidative stress led to increased membrane damage as assessed by live/dead assay and higher levels of potassium ion release from the cells treated with both silver nanoparticles and streptomycin. The results suggested that the combination of antibiotics with GK-AgNPs has an enhanced antibacterial action. Further, the GK-AgNPs were found to be biocompatible up to a concentration of 2.5 µg/mL as assessed with MTT assay on HeLa cell line. The results suggest that GK-AgNPs could potentially be used as in vivo antibacterial agent in combination with antibiotics to overcome the problem of antibiotic resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Resistance to antibiotics by pathogenic bacteria and fungi has been increasing at a disquieting rate and has become a serious problem, e.g., bacteria like E. coli and S. typhi have been reported to exhibit resistance to a variety of antibiotics (Wright 2005). Researchers worldwide have been trying to develop new, cost-effective and resistance-free effective antimicrobial agents, due to the prevalence of resistant or even multi-resistant pathogens and the continuing stress on health care costs (Gelatti et al. 2009). There are several examples of silver being widely used as an effective antimicrobial agent since ancient times (Rai et al. 2009). The antimicrobial activity of silver nanoparticles (AgNPs), in particular, is of significant interest because it was found to be effective against even multidrug resistance organisms (Ayala-Núñez and Villegas 2009). The silver nanoparticles are found to have broad spectrum activity and far lower propensity to induce microbial resistance than antibiotics (Inoue et al. 2002). These advantages have led to renaissance of AgNPs-based products like: wound dressings (Maneerung et al. 2008), antiseptic creams, water filters (Jain and Pradeep 2005), fabrics (Zhang et al. 2009), etc. However, toxicity issues have led to the limited use of bactericidal silver in nano form and needs to be investigated (Miura and Shinohara 2009). In this context, an alternative and intelligent approach is to effectively use AgNPs in combination with antibiotics so as to achieve desired antimicrobial potential at safe levels of both nano silver and antibiotics. In the recent past, many studies have been carried out to investigate the combinatorial/synergistic effect of AgNPs and antibiotics. It was reported that when amoxicillin and AgNPs are combined, it results in greater bactericidal efficiency on E.coli cells than when they were applied separately (Ping et al. 2005). In another study, the antibacterial activities of ampicillin, kanamycin, erythromycin, and chloramphenicol were found to increase in the presence of AgNPs against test strains (Fayaz et al. 2010). Similarly, the antifungal activity of fluconazole was enhanced in the presence of AgNPs against the test fungi (Gajbhiye et al. 2009). The main aim of this study is to investigate the combined effect of natural gum synthesized AgNPs with various antibiotics on both Gram classes of bacteria. In a previous study by our laboratory, we have reported a facile, eco-friendly method for the synthesis of gum kondagogu (Cochlospermum gossypium) reduced/stabilized silver nanoparticles (GK-AgNPs). The influence of different parameters: gum particle size, concentration of gum, concentration of silver nitrate and reaction time on the synthesis of nanoparticles was studied. At the optimized reaction conditions, it was possible to synthesize nearly monodispersed and size-controlled spherical AgNPs. Using well-diffusion assay, the antibacterial potential of GK-AgNPs was also demonstrated on both Gram classes of bacteria. This study reports the extent of GK-AgNPs synergy with various antibiotics.
Materials and methods
Materials
Gum kondagogu, grade-1 (Girijan Co-operative Corporation, Hyderabad, India), Silver nitrate (E. Merck, Mumbai, India), ciprofloxacin hydrochloride, streptomycin sulfate, gentamicin sulfate (Himedia, Mumbai, India), 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) (Sigma, Bengaluru, India) and LIVE/DEAD BacLight bacterial viability kit L7012 (Molecular probes, Eugene, USA) were used.
Synthesis and characterization of gum kondagogu reduced/stabilized silver nanoparticles (GK-AgNPs)
The GK-AgNPs were prepared as reported earlier by us (Kora et al. 2010). Briefly, dried gum kondagogu tears were powdered in a high-speed mechanical blender (Prestige, Bengaluru, India) and sieved to obtain a mean particle size of 38 µm. A 1.0 % (w/v) of homogenous gum stock solution was prepared in ultrapure water by stirring overnight at room temperature. The AgNPs were synthesized by autoclaving the 1 mM silver nitrate solutions containing 0.5 % of gum at 121 °C and 15 psi for 60 min. The synthesized GK-AgNPs were drop coated on a carbon-coated copper grid and subjected to transmission electron microscopy (TEM) (Philips CM200, The Netherlands) for determination of size and shape. The zeta potential of the nanoparticle solution was measured using a Malvern Zetasizer Nano ZS90 (Worcestershire, UK). The synthesized GK-AgNPs were used without any further purification.
Antibacterial assays
Micro-broth dilution assay
For determining the minimum inhibitory concentration (MIC) of GK-AgNPs and antibiotics, the micro-broth dilution method (Martinez-Castanon et al. 2008) was used. Staphylococcus aureus ATCC 25923 and Staphylococcus aureus ATCC 49834, and Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as model test strains for Gram-positive and Gram-negative bacteria, respectively. The assay was performed in a 96-well microtitre plate where the nutrient broth was supplemented with different concentrations of nanoparticles (1–10 µg/mL) and antibiotics: ciprofloxacin (0.05–0.5 µg/mL), streptomycin and gentamicin (0.1–5 µg/mL) followed by inoculation with bacterial cell suspension to obtain 106 CFU/mL. The control wells were maintained without any treatment. The MIC was determined after 48 h of static incubation at 37 °C by measuring the absorbance of these culture broths at 600 nm using a Biotek Synergy™ H1 plate reader (Bad Friedrichshall, Germany). To evaluate the synergism between GK-AgNPs and various antibiotics, the sub-MIC concentrations of both GK-AgNPs and antibiotics were added to the wells followed by inoculation with bacterial suspension of 106 CFU/mL. The occurrence and degree of synergism were checked by quantifying the fractional inhibitory concentration (FIC) index as reported earlier (Ghosh et al. 2013). The fractional inhibitory concentration (FIC) index = MIC of GK-AgNPs in combination with antibiotic/MIC of GK-AgNPs alone + MIC of antibiotic in combination with GK-AgNPs/MIC of antibiotic alone. If the FIC index ≤0.5, the effect is synergistic, while the value is 0.5 < FIC ≤ 1.0 it is additive and a value of FIC >1.0 is indifferent.
Assay for inhibition of biofilm formation
A modified microtitre plate assay (Uhlich et al. 2006) was performed to see the combinatorial effect of GK-AgNPs and antibiotics on biofilm formation. In brief, sterile, polystyrene microtitre plate wells were inoculated with 200 µL of nutrient broth containing 106 CFU/mL and treated with GK-AgNPs and antibiotics separately and in combination followed by incubation at 37 °C. The negative and positive controls were maintained with untreated and 70 % ethanol (50 µL/mL) treated cells, respectively. After 48 h of incubation, the medium in the wells was removed and washed with sterile phosphate-buffered saline (PBS) to remove the loosely attached bacteria. The wells were then stained with 200 µL of 0.1 % crystal violet for 30 min at 37 °C. Further, these wells were washed, air dried, the bound stain was solubilized in 200 µL of 95 % ethanol and the absorbance was recorded using a Biotek Synergy™ H1 micro-plate reader (Bad Friedrichshall, Germany) at 590 nm.
Measurement of intracellular reactive oxygen species (ROS)
For assessment of intracellular ROS (Choi and Hu 2008), exponentially grown cells were obtained by inoculating the overnight grown bacterial cell suspension (106 CFU/mL) in fresh nutrient broth and incubating at 37 °C, 65 rpm for 4 h. Then, the cells were collected by centrifugation (5,500×g, 10 min), followed by washing and resuspending in PBS. Further, the cells were labeled with 10 µM H2DCFDA (20 min, 37 °C) followed by treatment (1 h, 37 °C) with GK-AgNPs (2 µg/mL) and streptomycin (0.5 µg/mL). The negative and positive controls were maintained with untreated and 70 % ethanol (50 µL/mL) treated cells, respectively. The cells were washed with PBS and the fluorescence intensity was measured using a fluorescence plate reader with excitation and emissions at 490 and 520 nm, respectively.
Measurement of cytoplasmic potassium ion leakage
The K+ leakage analysis (Tiwari et al. 2008) of GK-AgNPs and streptomycin treated bacterial cells was done to evaluate the combined effect on bacterial membrane. The cells in exponential grown phase were obtained as detailed in “Measurement of intracellular reactive oxygen species (ROS)”. Later, the cells were treated with GK-AgNPs (2 µg/mL) and streptomycin (0.5 µg/mL) in PBS followed by incubation at 37 °C for 4 h. The negative and positive controls were maintained with untreated and 70 % ethanol (50 µL/mL) treated cells, respectively. The cells were centrifuged (5,500×g, 10 min) and the collected supernatants were analyzed for potassium content by a Jobin–Yvon Horiba JY-2000 ICP-OES (Longjumeau, France).
Live/dead assay
The synergistic effect of GK-AgNPs and streptomycin was also assessed with exponentially grown cells as described in “Measurement of intracellular reactive oxygen species (ROS)”, using Live/dead assay (Chamakura et al. 2011). For this, the P. aeruginosa 27853 cells were treated with GK-AgNPs (2 µg/mL) and streptomycin (0.5 µg/mL) for 4 h. Later, the cells were washed, resuspended in PBS and stained with LIVE/DEAD BacLight bacterial viability kit as per manufacturer’s instructions. The negative and positive controls were maintained with untreated and 70 % ethanol (50 µL/mL) treated cells, respectively. An aliquot of the stained cells was dropped on the glass slides and observed under LM-52-3001 Lawrence and Mayo epifluorescence microscope (Mumbai, India) under green filter. The digital images of the bacterial cells were captured using a Coolpix P6000 Nikon digital camera (Tokyo, Japan) attached to the microscope.
In vitro cytotoxicity assay
The HeLa cell cultures were maintained in DMEM with 10 % fetal calf serum. For cell toxicity tests, 2 × 105 cells at the exponential growth phase were seeded in each well of 96-well microtitre plates and incubated in an INC 108 Memmert CO2 incubator (Schwabach, Germany) (5 % CO2, 37 °C). A series of dilutions containing 1.0, 2.5, 5.0 and 10 µg/mL of GK-AgNPs were dispensed into the wells of microtitre plate. The negative and positive controls were maintained with untreated and doxorubicin (13 µg/mL) treated cells, respectively. The cell viability was measured at 48 h post-incubation using MTT (3-(4,5-dimethyathiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay. The assay was based on the measurement of the mitochondrial activity of viable cells by the reduction of tetrazolium salt to form a blue water-insoluble product, formazan (Cao et al. 2010). After 48 h of incubation, the MTT (5 mg/mL) was added to the cells and the plates were incubated for additional 4 h. Later, the medium was removed and 200 µL of DMSO was added to dissolve the formazan crystals. The absorbance of dissolved formazan was measured at 540 nm using a Biotek Synergy™ H1 plate reader (Bad Friedrichshall, Germany). The absorbance was directly correlated with the number of viable cells and was used to calculate the percentage viability.
Statistical analysis
Three independent experiments were carried out and the results are represented as mean ± SD. The Student’s t test was applied to calculate the statistical significance of the experimental data.
Results and discussion
Characterization of GK-AgNPs
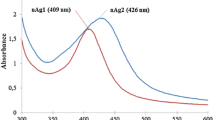
The TEM analysis revealed that the AgNPs synthesized with 0.5 % gum and 1 mM silver nitrate by 60 min of autoclaving; the nanoparticles synthesized were spherical in shape, with an average size of 5.8 ± 2.4 nm (Kora et al. 2010). The presence of concentric rings with intermittent bright dots in selected-area electron diffraction (SAED) pattern indicated the crystalline nature of the particles. The zeta potential of the synthesized nanoparticles was found to be −30.3 ± 4.1 mV, suggesting the stable nature of the GK-AgNPs (Fig. 1).
Micro-broth dilution assay
From the micro-broth dilution assay, the MIC of the AgNPs and antibiotics, streptomycin, gentamicin and ciprofloxacin, were calculated for both the Gram classes of bacteria (Table 1). For E. coli 25922, P. aeruginosa 27853, S. aureus 25923 and S. aureus 49834 strains, the MIC values of GK-AgNPs were 3.0, 5.0, 5.0 and 5.0 µg/mL, respectively. The results suggest that among the test strains, E. coli was found to be more sensitive towards the bactericidal activity of GK-AgNPs. The obtained MIC values were used as reference to calculate the FIC index of antibiotics in combination with GK-AgNPs (Table 1). It was observed that a concentration of 1 µg/mL of AgNPs in combination with either 0.25 µg/mL of streptomycin or 0.5 µg/mL of gentamicin or 0.1 µg/mL of ciprofloxacin was sufficient to inhibit more than 90 % of the bacterial growth in microtitre plates seeded with 106 CFU/mL, as compared to the control. For all the test strains, the FIC index of streptomycin indicated synergistic effect with GK-AgNPs. While the FIC values of gentamicin and ciprofloxacin in combination with GK-AgNPs, the effect was additive. Only with S.aureus 25923, the FIC index of gentamicin showed synergism with GK-AgNPs. Thus, the results indicate that the GK-AgNPs have either synergistic or additive effect with various antibiotics and the extent of effect depends both on the type of antibiotic and bacterial strain tested. These results are in concurrence with earlier studies carried out against spore-forming bacteria with combination of cinnamaldehyde with silver nanoparticles, in which at least an additive or near synergistic effect was observed (Ghosh et al. 2013). It is evident that among the antibiotics, streptomycin demonstrated highest activity against both the Gram classes of bacteria with AgNPs, in terms of synergism. Such an enhancement in antibacterial activity was earlier reported with streptomycin-loaded protein-capped gold nanoparticles (Rastogi et al. 2012) and collective activity of streptomycin and PVP-capped silver nanoparticles (Kora and Rastogi 2013).
The observations made in the present study have been compared with contemporary literature in relation to the enhanced antibacterial action of silver nanoparticles in combination with various antimicrobial agents. In an earlier synergistic antibacterial study with silver nanoparticles of 5–40 nm size, MIC values of 30 and 80 µg/mL were reported for E. coli and S. aureus strains, respectively. With disk diffusion assay against test strains, the antibacterial activities of ampicillin, kanamycin, erythromycin and chloramphenicol were increased in the presence of AgNPs (Fayaz et al. 2010). With the same test strains, the combined antibacterial activity of 16 nm sized PVP capped AgNPs with streptomycin, ampicillin and tetracycline was enhanced (Kora and Rastogi 2013). The combined efficacy of ciprofloxacin and gentamicin antibiotics with silver nanoparticles of 5–30 nm size was reported to be augmented against multidrug-resistant E. coli, P. aeruginosa and S. aureus strains (Naqvi et al. 2013). In another study using silver nanoparticles of 32.5 nm, the antifungal activity of fluconazole was enhanced in the presence of AgNPs against the test fungi (Fayaz et al. 2010). It is pertinent to note that all these studies were carried out with solid agar assays in contrast to broth-based assays used in the present study. In an earlier broth-based study carried out with AgNPs of 20 nm size, an MIC value of 40 µg/mL was reported and the combination of amoxicillin and AgNPs resulted in greater bactericidal efficiency against E.coli (Ping et al. 2005). The activity of 25–40 nm sized silver nanoparticles against E. coli, P. aeruginosa and S. aureus strains was greatly enhanced in micro-broth dilution method, when utilized in the presence of cinnamaldehyde, a food grade antimicrobial agent. For the respective strains, the reported MIC values of AgNPs were 0.6, 6.1 and 3.0 µg/mL. With same ATCC strains used in the present study; E. coli 25922, P. aeruginosa 27853 and S. aureus 25923, synergistic or near synergistic antibacterial activity of ampicillin, chloramphenicol and kanamycin were demonstrated with silver nanoparticles of 3 nm size and the reported MIC values AgNPs were 2.0, 0.5 and 0.5 µg/mL, respectively (Hwang et al. 2012).
The various degrees of planktonic growth inhibition induced by GK-AgNPs in combination with various antibiotics against used test strains are shown in Fig. 2. For E. coli 25922 and S. aureus 49834 strains, gentamicin and ciprofloxacin individually caused higher biofilm inhibition compared to the KG-AgNPs. Also with P. aeruginosa 27853 and S. aureus 25923, ciprofloxacin and gentamicin showed higher inhibition of biofilm formation than KG-AgNPs, respectively. Nevertheless, the combination of KG-AgNPs with all the antibiotics resulted in 95.2, 96.0, 95.5 and 96.2 % growth inhibition for E. coli 25922, P. aeruginosa 27853, S. aureus 25923 and S. aureus 49834, respectively.
Inhibition of biofilm formation
The results obtained from the micro-broth dilution assay were again confirmed from the biofilm inhibition assay against tested bacterial strains (Fig. 3). As shown in Fig. 2 that 1 µg/mL of AgNPs in combination with either 0.25 µg/mL of streptomycin or 0.5 µg/mL of gentamicin or 0.1 µg/mL of ciprofloxacin was able to significantly inhibit the biofilm formation in both Gram classes of bacteria. For Gram-negative E. coli, all the antibiotics individually caused higher biofilm inhibition compared to the KG-AgNPs. Also with P. aeruginosa 27853 and S. aureus 49834, gentamicin and ciprofloxacin showed higher inhibition of biofilm formation than KG-AgNPs. However, the combined activity of both the agents impeded ≥ 70 % of biofilm formation.
Generation of intracellular ROS
The antibacterial activity of AgNPs was found to be related to the formation of free radicals. Furthermore, the free radicals generated by the AgNPs induce bacterial cell membrane damage which is considered to be the main cause of cell death. Though the ROS can exist naturally in intracellular and extracellular locations, under certain conditions, high levels of ROS can cause oxidative stress in cells and lead to redox imbalance (Choi and Hu 2008). In this study, H2DCFDA was used to measure the intracellular ROS levels after various treatments (Fig. 4). For the cells treated with GK-AgNPs and streptomycin individually, a significant increase in ROS levels was observed as compared to the untreated cells. However, the cells treated with the combination of both GK-AgNPs and streptomycin showed even higher levels of ROS. The treatment of S. aureus 49834 cells individually with GK-AgNPs and streptomycin led to 2.0 and 1.1 fold increase in ROS generation, while a combined treatment with both the agents led to 3.0 fold increase in intracellular ROS levels. Similar pattern of ROS levels was observed for all other bacterial strains tested, though the fold increase in levels depended on the type of bacterial strain.
A bar graph showing the fluorescence intensities of various bacterial strains due to intracellular ROS generation after exposure with GK-AgNPs and streptomycin individually and in combination. Negative and positive controls denote untreated and 70 % ethanol-treated bacterial cell suspensions. Values are mean ± SD for n = 3; *p < 0.05, ***p < 0.001 and ****p < 0.0001 compared to negative control
Cytoplasmic potassium ion leakage
The bacterial membrane damage was further confirmed by quantifying the leakage of cytoplasmic potassium content. The leakage of cytoplasmic K+ has been correlated to the extent of membrane damage by several researchers (Tiwari et al. 2008). A significant increase in the levels of K+ was found in cells treated with GK-AgNPs for all the bacterial strains, when compared to negative and positive controls. But, the cells treated with streptomycin alone did not exhibit any considerable increase in the K+ levels. When the cells were exposed to both the GK-AgNPs and streptomycin, the K+ levels were higher than the cells treated with GK-AgNPs alone (Fig. 5). Though streptomycin alone was not able to cause much leakage of intracellular K+, still an increase in K+ leakage was observed when combined with AgNPs. The increased oxidative stress can cause damage not only to cell membrane, but also to proteins, DNA and components of respiratory system which in turn are responsible for augmented effect offered by GK-AgNPs and streptomycin combination.
The intracellular K+ leakage of test bacterial strains after treatment with GK-AgNPs and streptomycin individually and in combination. Negative and positive controls denote untreated and 70 % ethanol-treated bacterial cell suspensions. Values are mean ± SD for n = 3; *p < 0.05 and **p < 0.005 compared to negative control
Fluorescence microscopy
The damage to bacterial membrane was also estimated with Live/dead BacLight kit by differentiating the live and dead bacteria under fluorescence microscope. The viable cells with intact membrane were visualized by their green fluorescence under blue light and the dead cells with damaged membranes by their red fluorescence under green light. The fluorescence micrographs of P. aeruginosa 27853 control cells showed numerous intact green colored viable cells. Whereas, the cells treated with ethanol, GK-Ag-NPs and streptomycin showed several intense red colored cells. Further, the cells treated with both GK-AgNPs and streptomycin showed a higher population of red cells (Fig. 6). Thus, the fluorescence microscopic analyses demonstrate a higher cell wall and membrane damage, leading to an increase in cell death in bacteria treated with the combination of GK-Ag-NPs and streptomycin.
Evaluation of in vitro cytotoxicity
The biocompatibility of the silver nanoparticles was evaluated with HeLa cell line for their use in combination with antibiotics. HeLa cells were selected for cytotoxic studies as they are durable, prolific and can be grown very easily in 5 % serum-supplemented standard DMEM medium with no specific growth component requirement. The % viability of the cells was plotted against nanoparticles concentration at 48 h. The concentrations of nanoparticles up to 2.5 µg mL−1 were not cytotoxic. Beyond the concentration of 2.5 µg/mL, they were cytotoxic and the cell viability was reduced to 76.5 % and 18.9 % at 5 µg/mL and 10 µg/mL, respectively (Fig. 7). In the case of doxorubicin-treated positive control, the viability was reduced to 18.6 %. Thus, the data suggest that non-cytotoxic concentrations of antibacterial GK-AgNPs can be used for biomedical and therapeutic purposes (Ayala-Núñez and Villegas 2009).
Conclusions
In this study, we have demonstrated the synergistic and additive antibacterial effect of GK-AgNPs with aminoglycosidic and ciprofloxacin antibiotics, respectively, against both Gram classes of bacteria. It was found that the extent of enhancement was dependent on both bacterial strain and type of antibiotic in combination. The results may pave the way for the combined use of AgNPs and antibiotics towards decreasing the antibiotic resistance currently observed for certain bacteria. Research over last several years has confirmed that nanoscale materials can lead to unpredicted and abnormal toxicity and hence the dose is very crucial. Taking care of this point, by standardizing the concentration of antibiotics, we have shown more than 90 % of bacterial growth inhibition at non-cytotoxic levels of GK-AgNPs (1 µg/mL). Thus, in combination with antibiotics, the antibacterial efficiency and safety of GK-AgNPs were established for potential in vivo applications.
References
Ayala-Núñez NV, Villegas HHL et al (2009) Silver nanoparticles toxicity and bactericidal effect against methicillin-resistant Staphylococcus aureus: nanoscale does matter. Nanobiotechnology 5:2–9
Cao X, Cheng C, Ma Y, Zhao C (2010) Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. J Mater Sci 21:2861–2868
Chamakura K, Perez-Ballestero R, Luo Z, Bashir S, Liu J (2011) Comparison of bactericidal activities of silver nanoparticles with common chemical disinfectants. Colloid Surf B 84:88–96
Choi O, Hu Z (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42:4583–4588
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R (2010) Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine 6:103–109
Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M (2009) Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed Nanotechnol Biol Med 5:382–386
Gelatti LC, Bonamigo RR, Becker AP, dAzevedo PA (2009) Methicillin-resistant Staphylococcus aureus: emerging community dissemination. An Bra Dermatol 84:501–506
Ghosh IN, Patil SD, Sharma TK, Srivastava SK, Pathania R, Navani NK (2013) Synergistic action of cinnamaldehyde with silver nanoparticles against spore-forming bacteria: a case for judicious use of silver nanoparticles for antibacterial applications. Int J Nanomed 8:4721–4731
Hwang I-S, Hwang JH, Choi H, Kim K-J, Lee DG (2012) Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J Med Microbiol 61:1719–1726
Inoue Y et al (2002) Bactericidal activity of Ag-zeolite mediated by reactive oxygen species under aerated conditions. J Inorg Biochem 92:37–42
Jain P, Pradeep T (2005) Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol Bioeng 90:59–63
Kora AJ, Rastogi L (2013) Enhancement of antibacterial activity of capped silver nanoparticles in combination with antibiotics, on model Gram-negative and Gram-positive bacteria. Bioinorg Chem Appl 2013:1–7
Kora AJ, Sashidhar RB, Arunachalam J (2010) Gum kondagogu (Cochlospermum gossypium): a template for the green synthesis and stabilization of silver nanoparticles with antibacterial application. Carbohydr Polym 82:670–679
Maneerung T, Tokura S, Rujiravanit R (2008) Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym 72:43–51
Martinez-Castanon GA, Nino-Martinez N, Martinez-Gutierrez F, Martinez-Mendoza JR, Ruiz F (2008) Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res 10:1343–1348
Miura N, Shinohara Y (2009) Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem biophyl res comm 390:733–737
Naqvi SZH, Kiran U, Ali MI, Jamal A, Hameed A, Ahmed S, Ali N (2013) Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanomed 8:3187–3195
Ping L, Juan L, Changzhu W, Qingsheng W, Jian L (2005) Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnol 16:1912–1917
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Rastogi L, Kora AJ, Arunachalam J (2012) Highly stable, protein capped gold nanoparticles as effective drug delivery vehicles for amino-glycosidic antibiotics. Mater Sci Eng C 32:1571–1577
Tiwari DK, Behari J, Sen P (2008) Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Curr Sci 95:647–655
Uhlich GA, Cooke PH, Solomon EB (2006) Analyses of the red-dry-rough phenotype of an Escherichia coli O157:H7 strain and its role in biofilm formation and resistance to antibacterial agents. Appl Environ Microbiol 72:2564–2572
Wright GD (2005) Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv Drug Deliv Rev 57:1451–1470
Zhang F, Wu X, Chen Y, Lin H (2009) Application of silver nanoparticles to cotton fabric as an antibacterial textile finish. Fiber Polym 10:496–501
Acknowledgments
The authors would like to thank Dr. D. Karunasagar, Head, EACS and Dr. Sunil Jai Kumar, Head, NCCCM/BARC for their constant support and encouragement throughout the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Rastogi, L., Kora, A.J. & Sashidhar, R.B. Antibacterial effects of gum kondagogu reduced/stabilized silver nanoparticles in combination with various antibiotics: a mechanistic approach. Appl Nanosci 5, 535–543 (2015). https://doi.org/10.1007/s13204-014-0347-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0347-9