Abstract
Biosynthesis of silver nanoparticles (Ag Nps) was carried out using methanol leaves extract of L. reticulata. Ag Nps were characterized based on the observations of UV–visible spectroscopy, transmission electron microscopy, and X-ray diffraction (XRD) analysis. These Ag Nps were tested for antimicrobial activity by agar well diffusion method against different pathogenic microorganisms and antioxidant activity was performed using DPPH assay. Further, the in vitro cytotoxic effects of Ag Nps were screened against HCT15 cancer cell line and viability of tumor cells was confirmed using MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole)) assay. The nuclear condensation was studied using the propidium iodide-staining method. The color change from green to dark brown and the absorbance peak at about 420 nm indicated the formation of nanoparticles. XRD pattern showed characteristic peaks indexed to the crystalline planes (111), (200) and (220) of face-centered cubic silver. The nanoparticles were of spherical shape with varying sizes ranging from 50 to 70 nm. Biosynthesized Ag Nps showed potent antibacterial activity and effective radical scavenging activity. MTT assay revealed a dose-dependent decrease in cell viability. Microscopic observations showed distinct cellular morphological changes indicating unhealthy cells, whereas the control appeared normal. Increase in the number of propidium iodide positive cells were observed in maximum concentration. Methanolic leaf extract of L. reticulata acts as an excellent capping agent for the formation of silver nanoparticles and demonstrates immense biological activities. Hence, these Ag NPs can be used as antibacterial, antioxidant as well as cytotoxic agent in treating many medical complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In this era, nanotechnology is one of the most interesting areas which are used to describe the creation and utilization of materials with structural features between those of atoms and bulk materials with at least one dimension in the nano range. Nanoparticles are atomic or molecular aggregates with at least one dimension between 1 and 100 nm that can drastically modify their physico-chemical properties compared to the bulk material. It is worth noting that nanoparticles can be made from a full variety of bulk materials and that they can explicate their actions depending on both the chemical composition and on the size and/or shape of the particles (Brunner et al. 2006). Nanoparticles have been known to be used for numerous physical, biological, and pharmaceutical applications (Rai et al. 2009). Processes used for nanoparticles synthesis are chemical, physical, and a recently developed biological method. Chemical methods have various drawbacks including the use of toxic solvents, generation of hazardous by-products, and high energy consumption, which pose potential risks to human health and to the environment. Therefore, the biological method has an advantage over chemical and physical methods of nanoparticle synthesis, as it is cost-effective and environmentally friendly (Nabhikha et al. 2009). However, these methods also have the drawback of being rather slow (Balaji et al. 2009). The major biological systems involved in this are bacteria, fungi, and plant extracts (Prabhu and Poulose 2012). In recent years, the biosynthesis of nanoparticles using plant extracts has gained more importance. The synthesis and applications of silver nanoparticles from several plants have been studied by many researchers (Ankamwar et al. 2005; Kumar et al. 2010; Saxena et al. 2010; Ahmad et al. 2010). In most of the therapeutic applications, it is the antimicrobial property that is being majorly explored, though anti-inflammatory property, cytotoxicity and antioxidants have their fair share of applications.
Leptadenia reticulata (Retz) Wight & Arn. belonging to family Asclepiadaceae is an important endangered medicinal plant. In India it is found in Gujarat, Punjab, Himalayan ranges, Konkon, Nilgiris, and Southern part of India (Sudipta et al. 2011). The various reports on its multiple uses in curing several diseases such as hematopoiesis, emaciation, cough, dyspnoea, fever, burning sensation, and night blindness draw the attention for utilization of this plant as drugs. It is regarded as good cure for tuberculosis and effectively used for several ear and nose problems. 50 % methanolic extract of this plant is having antibacterial activity and is used for the treatment of skin infection and wounds (Sivarajan and Balachandran 1994). Anjaria in 1967 tried Leptaden tablets (a formulation from Leptadenia reticulata) on some clinical cases and reported its beneficial use as galactogogue for increasing milk (Anjaria and Gupta 1967). Hence in this study, for the first time we evaluated the synthesis and biological activities of Ag NPs using leaf extract of L. reticulata.
Materials and methods
Chemicals
The chemical silver nitrate (AgNO3), Mueller–Hinton agar (MHA), and Sabouraud dextrose agar (SDA) were purchased from Hi Media Laboratories Mumbai, India. Tissue culture plastic wares were obtained from Tarsons Products Pvt. Ltd., India. All organic solvents used were of HPLC grade. RPMI 1640, fetal bovine serum (FBS), and MTT (3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyltetrazolium bromide) were purchased from Sigma Aldrich, India.
Collection of plant material
Healthy, disease-free leaves of Leptadenia reticulata were collected during the month of April in 2013 from in and around the Padmahree Campus, Kommagatta, Sulikeri post of Bangalore, India. The collected leaves were washed thoroughly in tap water and then in detergent water and were finally rinsed with distilled water until no foreign material remained. The freshly cleaned leaves were left to dry in sun light for approximately 10 days.
Preparation of the plant extract
The leaves (20 g) were washed twice in tap water and rinsed thrice in distilled water. Then they were surface sterilized by HgCl2 (0.1 %) for 1 min, cut into small pieces, dried in the micro-oven, and ground into powder using an electronic blender. About 100 g of leaf powder material was uniformly packed into a thimble and run in soxhlet extractor. It was extracted with methanol for the period of about 5–6 cycles. After that extracts were filtered with the help of Whatman No. 1 filter paper. The filtrates were then evaporated under reduced pressure and dried using a rotary evaporator at 55 °C. Then the extract was kept in refrigerator at 4 °C for future experiments.
Synthesis of silver nanoparticles (Ag NPs)
For reduction of silver ions, 10 ml of collected filtrate was treated with 90 ml of silver nitrate aqueous solution (21.2 g of AgNO3 powder in 125 ml of Milli Q water) and incubated at room temperature for 10 min, resulting in the formation of yellowish to bright yellow and to dark brown color indicating the synthesis of silver nanoparticles (Parashar et al. 2009). After 8 h of incubation, the solution was centrifuged with 12,000 rpm for 20 min, and their pellets were redispersed in sterile distilled water. The centrifugation and redispersion were repeated three times to ensure the complete separation of nanoparticles. After drying, purified nanoparticles were resuspended in deionized water and stored in a freezer for further study.
Characterization of nanoparticles
UV–Vis spectroscopy
About 1 ml (diluted with 1:20 V/V Milli Q water) of plant-part-synthesised silver nanoparticle solution was monitored in UV–Vis spectrophotometer (Elico, India) (between 300 and 700 nm) with different time intervals (15 and 30 min; 4, 6, and 8 h).
Transmission electron microscopy (TEM)
Transmission electron microscope (TEM) analysis was done using JEM-1200EX electron microscope (JEOL, Japan). Thin films of sample were prepared on a carbon-coated copper grid by just dropping a very small amount of sample on the grid, extra solution was removed using a blotting paper, and then the film on the TEM grid were allowed to dry by putting it under incubator. In this technique, whereby a beam of electronics is transmitted through an ultra-thin specimen, interacting with the specimen as it passes through. An image is formed from the interaction of the electrons transmitted through the specimen. The image is magnified and focused on to an imaging device.
X-ray diffraction analysis (XRD)
XRD measurements of the silver nanoparticle solution drop-coated on glass were done on a XRD-6000 X-ray diffractometer model (Shimadzu, Japan) with 40 kV, 30 mA with Cu kα radiation at 2θ angle.
Evaluation of antibacterial activity
The silver nanoparticles (Ag NPs) synthesized using L. reticulata leaves extract were tested for antimicrobial activity by agar well diffusion method against different pathogenic microorganisms Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae) (Gram negative), Streptococcus pneumoniae (S. pneumoniae), Micrococcus luteus (M. luteus), and Bacillus subtilis (B. subtilis) (Gram positive). The pure cultures of bacteria were subcultured on MHA. Each strain was swabbed uniformly onto the individual plates using sterile cotton swabs. Wells of 8 mm diameter were made on nutrient agar plates using gel puncture. Using a micropipette, different concentrations (25, 50, 75, 100, 150 μg/ml) of nanoparticle solution was poured onto each well on all plates. After incubation at 37 °C for 24 h, the diameter of zone inhibition was measured in millimeter, and was recorded as mean ± SD of the triplicate experiments.
Studies on antioxidant activity
DPPH free radical scavenging assay
The DPPH free radical scavenging assay was conducted based on the method of (Chang et al. 2002; Dipankar and Murugan 2012). One milliliter of 0.1 mM DPPH (in ethanol) was added to different concentrations (100, 200, 300, 400, and 500 μg/ml) of methanolic leaf extract of L. reticulata and its Ag NPs. The reaction mixture was shaken and incubated in the dark for 30 min. The absorbance at 517 nm was measured against a blank (ethanol). Ascorbic acid was used as the standard and the control was prepared as described above without a sample. The lower absorbance of the reaction mixture indicated a higher percentage of scavenging activity. The percentage of inhibition or scavenging of free radicals was determined by the following formula:
Cytotoxity test of biosynthesized Ag NPs on human colon cancer cell line HTC15
Cell viability assay
HCT15 cell line was purchased from National Centre for Cell Science (NCCS), Pune, India. The cell line was grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 g/ml streptomycin, and 10 % fetal bovine serum (FBS). Cells were cultured in 75 cm2 cell culture flasks at 37 °C in a 5 % CO2 atmosphere. HCT15 cells were cultured and seeded into 96-well plates approximately as 5 × 104 cells in each well and incubated for 48 h. HCT15 cells were treated with different concentrations of synthesized Ag NPs (10, 20, 50, 70, 100 g/ml). After treatment, the plates were incubated for 24–48 h to perform cytotoxic analysis using MTT assay. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole) was prepared at a concentration of 5 mg/ml, and 10 ml of MTT was added to each well and incubated for 4 h. Purple color formazone crystals that formed were then dissolved in 100 ml of dimethyl sulphoxide (DMSO). These crystals were observed at 570 nm in a multi well ELISA plate reader. Optical density value was subjected to percentage of viability using the following formula:
Cytomorphology observation
The cells were seeded at 1 × 105 cells/well into a six-well chamber plate and incubated overnight. Later, the medium was replaced with maintenance medium DMEM without FBS containing 20 g/ml Ag NPs incubated for 48 h. The cytomorphology was examined under Nikon inverted microscope.
Propidium iodide staining
HCT15 cells were plated at 5 × 104 cells/well into a six-well chamber plate. At >90 % confluence, the cells were treated with Ag NPs for 48 h. The cells were washed with PBS fixed in methanol: acetic acid (3:1, v/v) for 10 min and stained with 50 g/ml Propidium iodide for 20 min. Nuclear morphology of apoptotic cells with condensed/fragmented nuclei was examined under Confocal microscope.
Statistical analysis
The grouped data were statistically evaluated using Microsoft excel worksheet. Values are presented as the mean ± SD of the three replicates of each experiment.
Results and discussion
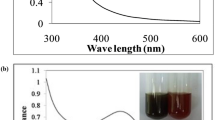
The present study was carried out to bio synthesize Ag NPs using leaf extract of L. reticulata and to study their biological properties. Nanoparticles are generally characterized by their size, shape, surface area, and dispersity. Homogeneity of these properties is important in many applications (Jiang et al. 2009). When the leaf extract was mixed with AgNO3 and incubated at room temperature, within 1 h of the reaction, its color changed from green to dark brown (Fig. 1), indicating the formation of Ag NPs. It is an efficient and rapid method, which was very well explained by other researchers who worked with different plant systems (Chandran et al. 2006; Li et al. 2007; Shankar et al. 2004). Change in color was due to the excitation of surface plasmon vibrations in the metal nanoparticles (Ahmad et al. 2003). Our results are in conformity with Bonde et al. (2012), who reported the formation of Ag NPs within 1 h of incubation. However, Dipankar and Murugan (2012) reported color change after 2–3 days indicating the slow reduction of the AgNO3 by the aqueous leaf extract of Iresine herbstii. An extract of Ocimum sanctum leaves was reported to reduce silver ions to nanoparticles within 8 min (Mallikarjun et al. 2011). The dissimilarity in the rates of bio-reduction observed may be due to the differences in the activities of the enzymes present in the plant leaf extracts.
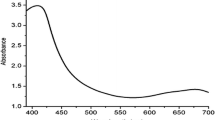
It is generally recognized that UV–Vis spectroscopy could be used to examine size- and shape-controlled nanoparticles in aqueous suspensions. In order to verify the synthesis of Ag NPs, the test samples were subjected to UV–Vis spectrophotometric analysis (Fig. 2). This analysis showed the sharp absorbance at around 450 nm (Fig. 2), which was specific for Ag NPs. The UV–Vis absorption band in the current visible light region (425–460 nm) is an evidence of the presence of surface plasmon resonance (SPR) of silver nanoparticles (Ankamwar et al. 2005; Kumar et al. 2010; Saxena et al. 2010; Mallikarjun et al. 2011; Bonde et al. 2012). The reduction was ascribed to the phenolics, terpenoids, polysaccharides, and flavones compounds present in the extract (Huang et al. 2007). The X-ray diffraction pattern of the biosynthesised Ag NPs from the leaf extract is shown in Fig. 3. XRD pattern of the biosynthesized Ag NPs showed characteristic peaks indexed to the crystalline planes (111), (200) and (220) of face-centered cubic silver. The values were in agreement with the findings of Joint Committee on powder diffraction (JCPD, standard file no. 04-0783). The sharpness of the peak clearly indicates that the particles are in the nanoregime. XRD pattern thus clearly illustrates that the silver nanoparticles formed in this present synthesis are crystalline in nature (Harekrishna et al. 2009). The applications of silver nanoparticles are dependent on their size and shape (Sivaraman et al. 2009). To get more evidence the s shape and size of Ag NPs were analyzed after 24 h of incubation using TEM (Fig. 4). In general, the nanoparticles were in spherical shape with varying sizes ranging from 50 to 70 nm. One should note that TEM images were made by attaching a single drop of the nanoparticle solution onto a carbon film. Hence the single drop used for each sample cannot fully represent the entire solution. Also the use of the electron beam in acquiring the TEM image may somewhat alter the arrangement of the nanoparticles (Popov et al. 2006). In our study, TEM images were verified with multiple trials and evidenced that there is variation in particle sizes. However, we found very little variations in the sizes of the nanoparticles (50–70 nm). It is observed that most of the Ag NPs were of various spherical shapes, which fall closer to many of the silver nanoparticles produced by other plant materials (Shankar et al. 2004; Huang et al. 2007; Ali et al. 2011).
The present study clearly suggests the potential antibacterial activities of Ag NPs synthesized by methanol leaves extract of L. reticulata (Fig. 5). The results showed that antibacterial activity was dose dependant (Table 1). The maximum zone of inhibition with the B. subtilis (24.3 ± 1.3 mm), followed by E. coli (20.0 ± 0.4 mm), was noticed at 150 μg/ml concentration of Ag NPs. The lowest zone of inhibition was observed with the M. luteus (08.7 ± 1.4 mm) at 25 μg/ml concentration of Ag NPs. Irrespective of the microbes tested, zone of inhibition increased with increased concentration of Ag NPs. Similarly, Dipankar and Murugan (2012) have reported dose-dependant inhibition by Ag NPs synthesized from Iresine herbstii leaf aqueous extracts. This might be due to denaturation of bacterial cell wall, blocking bacterial respiration, destabilization of outer membrane, and depletion of intracellular ATP (Maliszewska and Sadowski 2009). Ag NPs were more effective against Gram-positive bacterial strains than the Gram-negative bacteria strains. Further, the variation of the sensitivity between Gram-positive and Gram-negative bacterial isolates might be attributed by the membrane permeability (Ravikumar et al. 2010).
Antioxidants are compounds that protect cells against damaging effects of reactive oxygen species which can neutralize free radicals before they can do harm and undo some damage already caused to specific cells. Many medicinal plants are known to possess free radical scavenging molecules, such as phenolic compounds, terpenoids, vitamins, and some other endogenous metabolites, that are rich in antioxidant activity (Koleva et al. 2002). In the present study, antioxidant activity of the methanol extract of L. reticulata leaf and its Ag NPs were investigated by DPPH scavenging assay. DPPH stable free radical method is an easy, rapid and sensitive way to survey the antioxidant activity of a specific compound or plant extracts (Koleva et al. 2002). The results revealed the existence of effective radical scavenging activity of both plant extract and biosynthesized Ag NPs when compared with the standard, ascorbic acid (Fig. 6). The highest radical scavenging activity with 64.81 % was observed in Ag NPs at 500 μg/ml. The DPPH radical scavenging activities of plant extract and Ag NPs increased gradually in a dose-dependent manner. Similar results are obtained by Dipankar and Murugan (2012) in I. herbstii aqueous leaf extracts. However, Ag NPs exhibited higher inhibition rate when compared to leaf extract, suggesting the potential benefits of Ag NPs as antioxidative agents.
The in vitro cytotoxic effects of silver nanoparticles were screened against HCT15 cancer cell line and viability of tumor cells was confirmed using MTT assay. The biosynthesized Ag NPs from were evaluated for their effect on cell viability against HCT15 cancer cell line at different concentrations. The biosynthesized Ag NPs showed a dose-dependent decrease in cell viability. Increasing the time of incubation showed a further decrease in cell viability. The results suggested that the synthesized Ag NPs were very sensitive (Fig. 7). IC 50 value was found to be 20 μg/ml at 48 h of incubation. Microscopic observations were monitored using Nikon light inverted microscope wherein treated cells showed distinct cellular morphological changes indicating unhealthy cells, whereas the control appeared normal (Fig. 8). Control cells were irregular confluent aggregates with rounded and polygonal cells. Biosynthesized Ag NPs treated cells appeared to shrink and became spherical in shape, and cell spreading patterns were restricted when compared to control. To confirm whether the cytotoxic effect induced by Ag NPs biosynthesized from leaves extract of L. reticulata involves apoptotic changes, the nuclear condensation was studied by the propidium iodide staining method. In the case of control cells, a very negligible number of propidium iodide-positive cells were present. The cells treated with 20 µg/ml of biosynthesized Ag NPs showed more number of propidium iodide-positive cells (Fig. 9). The cytotoxic effects of silver are the result of active physicochemical interaction of silver atoms with the functional groups of intracellular proteins, as well as with the nitrogen bases and phosphate groups in DNA (Moaddab et al. 2011). There are only few studies on the cytotoxic effect of plant-based Ag NPs against Hep 2 cell line, HeLa cell line, HL 60 cell line (Rosarin et al. 2013; Sulaiman et al. 2013). However, our study is the first report on the use of HCT15 cancer cell line. Hence, this study will help in knowing about the potential benefits of L. reticulata Ag NPs for treating cancer. However, further studies on the mechanisms of action of Ag NPs on cytotoxicity are required to rate the risks and benefits of these plant-based nanoparticles.
Conclusion
In conclusion, the leaf extract of L. reticulata can be used efficiently to produce Ag NPs. This is a simple, eco-friendly process and has potent applications in biomedical and pharmaceutical applications. The bio-reduction observed may be due to the activities various plant metabolites present in the leaf extracts. XRD pattern thus clearly illustrates that the silver nanoparticles formed in this present synthesis are crystalline in nature. The nanoparticles were spherical in shape with varying sizes ranging from 50 to 70 nm. The biosynthesized Ag NPs demonstrated an excellent antibacterial activity, antioxidant activity, and cytotoxic activity. Hence, Ag NPs can be explored as a new source of alternative medicine for treating many human ailments.
References
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B 28:313–318
Ahmad N, Sharma S, Alam MK, Singh VN, Shamsi SF, Mehta BR (2010) Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloid Surf B 81:81–86
Ali DM, Thajuddin N, Jeganathan K, Gunasekaran M (2011) Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B Biointerfaces 85:360–365
Anjaria JV, Gupta I (1967) Studies on lactogenic property of Leptadenia reticulata and Leptaden tablet in goats, sheep, cows and buffaloes. Indian Vet J 44:967–974
Ankamwar B, Chaudhary M, Sastry M (2005) Biosynthesis of gold and silver nanoparticles using Emblicaofficinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol 5:1665–1671
Balaji DS, Basavaraja S, Deshpande R, Mahesh DB, Prabhakar BK, Venkataraman A (2009) Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B 68(1):88–92
Bonde SR, Rathod DP, Ingle AP, Ade RB, Gade AK, Rai MK (2012) Murraya koenigii—mediated synthesis of silver nanoparticles and its activity against three human pathogenic bacteria. Nanosci Methods 1:25–36
Brunner TI, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ (2006) In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and effect of particle solubility. Env Sci Technol 40:4374–4381
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanotriangles using Aloe vera plant extract. Biotechnol Prog 22:577–579
Chang W, Choi SCK, Soon SH, Bong KC, Hye JA, Min YL, Sang HP, Soo KK (2002) Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay guided comparison. Plant Sci 163:1161–1168
Dipankar C, Murugan S (2012) The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf B 98:112–119
Harekrishna B, Dipak KB, Gobinda PS, Priyanka S, Sankar PD, Ajay M (2009) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A Physicochem Eng Asp 339:134–139
Huang J, Chen C, He N, Hong J, Lu L, Qingbiao L, Shao W, Sun D, Wang XH, Wang Y, Yiang X (2007) Biosynthesis of silver and gold nanoparticles by novel sun dried Cinnamomum camphora leaf. Nanotechnology 18:105–106
Jiang J, Oberdörster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11:77–89
Koleva II, Van Beek TA, Linssen JPH, de Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 13:8–17
Kumar V, Yadav SC, Yadav SK (2010) Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J Chem Technol Biotechnol 85:1301–1309
Li S, Shen Y, Xie A, Yu X, Qiu L, Zhang L, Zhang Q (2007) Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem 9:825–858
Maliszewska I, Sadowski Z (2009) Synthesis and antibacterial activity of of silver nanoparticles. J Phys Conf Ser 146(1) (Article ID 012024)
Mallikarjun K, Narsimha G, Dillip G, Praveen B, Shreedhar B, Lakshmi S (2011) Green synthesis of silver nanoparticles using Ocimum leaf extract and their characterization. Dig J Nanomater Biostruct 6:181–186
Moaddab S, Ahari H, Shahbazzadeh D, Motallebi AA, Anvar AA, Rahman-Nya J, Shokrgozar MR (2011) Toxicity study of nanosilver (Nanocid) on osteoblast cancer cell line. Int Nano Lett 1:11–16
Nabikha K, Kathiersan A, Raj M, Alikunhi N (2009) Synthesis of antimicrobial silver nanoparticles by callus and leaf extract from salt marsh plants, Sesuvium portulacastrum L. Colloids Surf Biointerfaces 79:488–493
Parashar UK, Saxenaa P, Srivastava A (2009) Bioinspired synthesis of silver nanoparticles. Dig J Nanomater Biostruct 4:159–166
Popov AK, Brummer J, Tanke RS, Taft G, Loth M, Langlois R, Wruck A, Schmitz R (2006) Synthesis of isolated silver nanoparticles and their aggregates manipulated by light. Laser Phys Lett 3(11):546–552
Prabhu S, Poulose EK (2012) Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2:32
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Ravikumar S, Gnanadesigan M, Suganthi P, Ramalakshmi A (2010) Antibacterial potential of chosen mangrove plants against isolated urinary tract infectious bacterial pathogens. Int J Med Med Sci 2(3):94–99
Rosarin FS, Arulmozhi V, Nagarajan S, Mirunalini S (2013) Antiproliferative effect of silver nanoparticles synthesized using amla on Hep2 cell line. Asian Pac J Trop Med 6(1):1–10
Saxena A, Tripathi RM, Singh RP (2010) Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig J Nanomater Bios 5:427–432
Shankar SS, Ahmad A, Rai A, Sastry M (2004) Rapid synthesis of Au, Ag and bimetallic Au core-Ag shell nanoparticles by using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 275:496–502
Sivarajan VV, Balachandran I (1994) Ayurvedic drugs and their plant sources. Oxford IBH Co. Pvt Ltd, India
Sivaraman SK, Elango I, Kumar S, Santhanam V (2009) A green protocol for room temperature synthesis of silver nanoparticles in seconds. Curr Sci 97(7):1055
Sudipta KM, KumaraSwamy M, Balasubramanya S, Anuradha M (2011) Cost effective approach for in vitro propagation of (Leptadenia reticulata Wight & Arn.)—a threatened plant of medicinal importance. J Phytol 3(2):72–79
Sulaiman GM, Mohammed WH, Marzoog TR, Al-Amiery AA, Kadhum AA, Mohamad AB, Bagnati R (2013) Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract. Asian Pac J Trop Biomed 3(1):58–63
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kumara Swamy, M., Sudipta, K.M., Jayanta, K. et al. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl Nanosci 5, 73–81 (2015). https://doi.org/10.1007/s13204-014-0293-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0293-6