Abstract
CdS is one of the most important II–VI semiconductors with applications in solar cells, optoelectronics and electronic devices. CdS nanoparticles were synthesized by the wet chemical method. The crystal structure and grain size of the particles were determined by X-ray diffraction. The optical properties were studied by the ultraviolet–visible absorption spectrum. The dielectric properties of CdS nanoparticles were studied in the frequency range of 50 Hz–5 MHz at different temperatures. The frequency dependence of the dielectric constant and dielectric loss is found to decrease with an increase in the frequency at different temperatures. The dielectric properties of CdS nanoparticles are found to be significantly enhanced specially in the low frequency range due to confinement. Further, electronic properties, such as valence electron plasma energy, average energy gap or Penn gap, Fermi energy and electronic polarizability of the CdS nanoparticles were calculated. The AC electrical conductivity measurements reveal that the conduction depends on both the frequency and the temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanomaterials have attracted interest and attention due to their special characteristics that differ from those of bulk solids and molecules. Because the novel properties of nanomaterials depend on their size, structure and shape, an understanding of their mechanism as well as a new direction for synthetic methods are significant issues in nanoscience and nanotechnology (White et al. 2009). As a result of the high surface-to-volume ratio of the nanoparticles, the surface properties have significant effects on their structure, and in turn, have an influence on the nanoscale devices in electronics to target in drug delivery (Hullavarad et al. 2008). In recent years, semiconductor nanoparticles exhibit specific properties due to the quantum confinement effects. As a consequence of their size in the nanometric range and the special luminescent properties, a widening of the band gap is caused when the spatial dimension is reduced (Brus 1986; Rossetti et al. 1985; Hu and Zhang 2006). In semiconductors, the quantum confinement modulates the band structure of the nanoparticles, and hence, many properties can be tuned by changing the nanoparticles size. Owing to the quantum size effect of the semiconductor nanoparticles, the II–VI semiconductor nanoparticles in particular, exhibit size-dependent optical properties (Alivisatos 1996), which are of great importance for potential applications, such as light emitting diodes (Coe et al. 2002), biological labels (Zhang et al. 2003; Sato et al. 2008), optoelectronic devices (Duan et al. 2003) and solar cells (Huynh et al. 2002). Research in semiconducting nanoparticles is one of the most investigated subjects, due to their wide field of applications. Recently, the surface modification of fluorescent semiconductor nanoparticles by biomolecules and nucleic acids, has added a new dimension to nanoparticle research, with respect to their biological applications (Mamedova et al. 2001). This article deals with the preparation of CdS nanoparticles using the wet chemical method. The prepared nanoparticles were characterized structurally and optically using powder the XRD and the UV–Vis absorption spectrum. The dielectric studies have been carried out in the frequency range 50 Hz–5 MHz at different temperatures. Some of the electronic properties, such as plasma energy, Penn gap, Fermi energy and electronic polarizability of the CdS nanoparticles were determined.
Experimental procedure
Nanoparticles of CdS were synthesized by the wet chemical technique, starting from cadmium nitrate and sodium sulfide precursors. Glucose was used as a capping agent. After 12 h of constant stirring, a pale yellow coloured solution was obtained. The precipitates were washed several times with ethanol and centrifuged and dried at 50 °C for 3 h in vacuum. The structure and phase of the samples were determined by the XRD with CuKα radiation (λ = 1.5481 nm); the 2θ range used was from 10° to 70° at a scanning rate of 0.02 °/s. The UV–visible absorption spectrum of synthesised CdS nanoparticles was recorded using the Varian Cary Model 5000 spectrophotometer in the wavelength range of 400–700 nm. The dielectric constant and the dielectric loss of the pellets of CdS nanoparticles in disk form were studied at different temperatures using an HIOKI 3532 LCR HITESTER in the frequency region of 50 Hz–5 MHz.
Results and discussion
Xrd
The XRD pattern of CdS nanoparticles is shown in Fig. 1. The XRD peaks are found to be broad which indicate the fine sized grain. The XRD pattern exhibits the prominent broad peaks at 2θ values of 26.80°, 43.90° and 52.20°. The synthesized nanoparticles have good crystallinity, and the average particle size obtained using the diffraction pattern was found to be 3.85 nm.
UV absorption
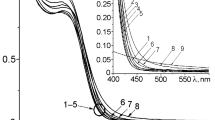
The UV–visible absorption spectroscopy has been used to focus the optical properties of quantum-sized particles. The absorption spectrum of the nanoparticles of CdS is shown in Fig. 2. The systematic shift in the absorption edge with changing particle size is obviously due to the quantum size effect of the CdS nanoparticles. It is interesting to note that a strong absorption band at 480 nm is observed in Fig. 2, which indicates the narrow distribution of the CdS nanoparticles. It is well known that the diameter of the particles is related to the absorption edge (Moffitt and Eisenberg 1995; He et al. 2003). Generally, the wavelength of the maximum exciton absorption decreases as the particle size decreases, as a result of the quantum confinement of the photo generated electron–hole pairs. The energy bandgap of these synthesized nanoparticles was estimated using the relation,
where Eg, is the bandgap, h, is Planck’s constant, c, is the velocity of light, and λmax, is the maximum absorption of CdS nanoparticles. The value of the bandgap is found to be 2.58 eV. The absorption band edge was shifted to 480 nm and the corresponding bandgap is 2.58 eV which is higher as compared to the bulk CdS bandgap (2.40 eV).
Dielectric properties
The dielectric constant and the dielectric loss of the CdS nanoparticles were studied at different temperatures using the HIOKI 3532 LCR HITESTER in the frequency region 50 Hz–5 MHz. The dielectric constant was measured as a function of the frequency at different temperatures as shown in Fig. 3, while the corresponding dielectric losses are depicted in Fig. 4. The dielectric constant is evaluated using the relation,
where d is the thickness of the sample and A, is the area of the sample. Figure 3 shows the plot of the dielectric constant (εr) versus applied frequency. It is observed (Fig. 3) that the dielectric constant decreases exponentially with increasing frequency and then attains almost a constant value in the high frequency region. This also indicates that the value of the dielectric constant increases with an increase in the temperatures. The net polarization present in the material is due to ionic polarization, electronic polarization, dipolar polarization and space charge polarization. The contribution of the decrease in the dielectric constant due to electronic polarization is quite less. Dipolar polarization is also expected to decrease with temperature as it is inversely proportional to temperature. The contribution to polarizability of the space charge depends on the purity of the nanoparticles. At low temperature and high frequency, we may take it as negligible. However, it is significant in the low frequency region. As the temperature increases, the contribution of the space charge effect towards polarization may have a tendency to increase. The dielectric loss studied as a function of frequency at different temperatures is shown in Fig. 4. These curves suggest that the dielectric loss is strongly dependent on the frequency of the applied field, similar to that of the dielectric constant. The dielectric loss decreases with an increase in the frequency at almost all temperatures, but appears to achieve saturation in the higher frequency range of 1 MHz and above, at all the temperatures. In the low frequency region, high energy loss is observed, which may be due to the dielectric polarization, space charge and rotation direction polarization occurring in the low frequency range. The value of the dielectric loss is found to be higher than that of the bulk CdS in the low frequency region. The high value of the dielectric constant at low frequencies may be due to the presence of all the four polarizations; namely, space charge, orientation, electronic and ionic polarization and its low value at higher frequencies may be due to the gradual loss of significance of these polarizations (Smyth 1965). This may lead to large values of dielectric constant at low frequencies. The CdS pellets exposed to an external electric field, positive and negative charges on interfaces moves towards the negative and positive poles of the electric field, respectively. Meanwhile, a great number of dipole moments are unavoidably formed after they have been trapped by defects. Consequently, space–charge polarization occurs in the interfaces of the CdS nanoparticles, which results in the much larger dielectric constant of the CdS nanoparticles. For conventional CdS powders, it is impossible to observe space-charge polarization due to the smaller specific surface. The dielectric constant value of the CdS nanoparticles is found to be very high compared to that of the bulk CdS, which is in good agreement with the earlier reports (Abdulkhadar and Thomas 1995).
In the proposed relation only one parameter, such as the high-frequency dielectric constant is required as input, to evaluate electronic properties like valence electron plasma energy, average energy gap or Penn gap, Fermi energy and electronic polarizability of the CdS nanoparticles were calculated. The theoretical calculations show that the high-frequency dielectric constant is explicitly dependent on the valence electron Plasmon energy, an average energy gap referred to as the Penn gap and Fermi energy. The Penn gap is determined by fitting the dielectric constant with the Plasmon energy (Ravindra et al. 1981). The valence electron plasma energy, \( \hbar \omega_{\rm p}, \) is calculated using the relation (Kumar and Sastry 2005),
According to the Penn model (Penn 1962), the average energy gap for a CdS nanoparticles is given by
where \( \hbar \omega_{P} \) is the valence electron plasmon energy and the Fermi energy (Ravindra et al. 1981) given by
Then we obtained electronic polarizability α, using a relation (Ravindra and Srivastava 1980; Penn 1962),
where S0 is a constant given by
The value of α obtained from Eq. (6) closely matches with that obtained using the Clausius–Mossotti relation,
When considering that the polarizability is highly sensitive to the bandgap (Reddy et al. 1995), the following empirical relationship is also used to calculate α,
where Eg is the bandgap value determined through UV absorption spectrum. The high frequency dielectric constant of materials is very important parameter for calculating the physical or electronic properties of materials. All the above parameters as estimated are shown in Table 1.
AC electrical conductivity studies
The temperature dependent AC electrical conductivity measurement was carried out. The AC conductivity (σ) of the CdS nanoparticles was calculated using the relation,
The σ values were fitted into the equation, \( \sigma = \sigma_{0} \exp [ - E/kT] \) (where, k is the Boltzmann constant, T is the absolute temperature and σ0 is a constant depending on the material) and the activation energy E was estimated (Kurien et al. 2006). The temperature dependent AC conductivity of the CdS nanoparticles is shown in Fig. 5. It is observed that the conductivity (σ) increases with the temperatures. The activation energy of the CdS nanoparticles is found to be 0.12 eV.
Conclusion
Nanoparticles of CdS are synthesized using the wet chemical method. The crystal structure and grain size of the particles are determined using the XRD studies and the particle size of the CdS nanoparticle is found to be 3.85 nm. From the optical absorption spectrum, it is seen that the blue shift of 480 nm with respect to the bulk counterpart is contributed by the quantum confinement effect. The value of the bandgap is found to be 2.58 eV. The dielectric constant and dielectric loss of the CdS nanoparticles are measured in the frequency range of 50 Hz–5 MHz at different temperatures. Dielectric studies reveal that both the dielectric constant and dielectric loss decrease with an increase in the frequency. The dielectric characterization shows the low value of the dielectric constant at higher frequencies. The dielectric constant of CdS nanoparticles is found to be much larger than that of the bulk CdS. Some of the electronic properties, such as the plasma energy, Penn gap, Fermi energy and electronic polarizability of the CdS nanoparticles have been calculated. AC electrical conductivity was found to increase with an increase in the temperatures and frequency. The results reveal that the AC electrical conductivity varies almost linearly with the applied frequency in the high range and increases with different temperatures.
References
Abdulkhadar M, Thomas B (1995) Study of dielectric properties of nano-particles of cadmium sulphide. Phys Stat Sol A 150:755
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271:933–937
Brus LE (1986) Electron wave function in semiconductor clusters: experiment and theory. J Phys Chem 90:2555–2560
Coe S, Woo WK, Bawendi MG, Bulovic V (2002) Electroluminescence from single monolayers of nanocrystals in molecular organic devices. Nature 420:800–803
Duan XF, Huang Y, Agarwal R, Lieber CM (2003) Single-nanowire electrically driven lasers. Nature 421:241–245
He R, Qian X, Yin J, Xi H, Bian L, Zhu Z (2003) Formation of monodispersed PVP-capped ZnS and CdS nanocrystals under microwave irradiation. Colloids Surf A 220:151–157
Hu H, Zhang W (2006) Synthesis and properties of transition metals and rare-earth metals doped ZnS nanoparticles. Opt Mater 28:536–550
Hullavarad NV, Hullavarad SS, Karulkar PC (2008) Review of Cadmium Sulfide CdS Nanotechnology: synthesis and Applications. J Nanosci Technol 8:3272–3299
Huynh WU, Dittmer JJ, Alivisatos AP (2002) Hybrid nanorod-polymer solar cells. Science 295:2425–2427
Kumar V, Sastry BSR (2005) Heat of formation of ternary chalcopyrite semiconductors. J Phys Chem Solids 66:99–102
Kurien S, Mathew J, Sebastian S, Potty SN, George KC (2006) Dielectric behaviour and ac electrical conductivity of nanocrystalline nickel aluminate. Mater Chem Phys 98:470–476
Mamedova NN, Kotov NA, Rogach AL, Studer J (2001) Albumin–CdTe nanoparticle bioconjugates: preparation, structure, and interunit energy transfer with antenna effect. Nano Lett 1:281–286
Moffitt M, Eisenberg A (1995) Size control of nanoparticles in semiconductor polymer composites. 1. Control via multiplet aggregation numbers styrene base random ionomers. Chem Mater 7:1178–1184
Penn DR (1962) Wave-Number-Dependent Dielectric Function of Semiconductors. Phys Rev 128:2093–2097
Ravindra NM, Srivastava VK (1980) Properties of liquid lead monosulfiede, lead selenide and lead telluride. Infrared Phys 20:399–418
Ravindra NM, Bharadwaj RP, Sunil Kumar K, Srivastava VK (1981) Model based studies of some optical and electronic properties of narrow and wide gap materials. Infrared Phys 21:369–381
Reddy RR, Nazeer Ahammed Y, Ravi Kumar M (1995) Variation of magnetic susceptibility with electronic polarizability in compound semiconductors and alkali halides. J Phys Chem Solids 56:825–829
Rossetti R, Hull R, Gibson JM, Brus LE (1985) Excited electronic states and optical spectra of ZnS and CdS crystallites in the 15 to 50 A size range: evolution from molecular to bulk semiconducting properties. J Chem Phys 82:552–559
Sato K, Tachibana Y, Hattori S, Chiba T, Kuwabata S (2008) Polyacrylic acid coating of highly luminescent CdS nanocrystals for biological labelling applications. J Colloid Interface Sci 324:257–260
Smyth CP (1965) Dielectric Behavior and Structure. McGraw-Hill, New York, p 132
White RJ, Luque R, Budarin VL, Clark JH, Macquarrie DJ (2009) Supported metal nanoparticles on porous materials. Methods and applications. Chem Soc Rev 38:481–494
Zhang H, Wang LP, Xiong HM, Hu LH, Yang B, Li W (2003) Hydrothermal synthesis for high quality CdTe QDs. Adv Mater 15:1712–1715
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Suresh, S. Studies on the dielectric properties of CdS nanoparticles. Appl Nanosci 4, 325–329 (2014). https://doi.org/10.1007/s13204-013-0209-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0209-x