Abstract
Nanometer nickel phosphide compounds (Ni2P and Ni12P5) were synthesized via a mild hydrothermal method with red phosphor and nickel chloride as raw materials. XRD, EDS, TEM and SEM analysis were employed to characterize the obtained products. The results showed that the as-prepared products were well crystallized and particle sizes ranged from 10 to 40 nm. Effects of raw material ratios and initial pH of reaction system on the final products were investigated. The result showed that increased P/Ni ratio benefited the formation of Ni2P but went against obtaining Ni12P5 and nanoparticles were obtained only in alkaline environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owning to the great application prospects as catalytic agent (Oyama 2003; Muettcrties and Sauer 1974), synthesis of nickel phosphide compounds has been of great interest to researchers. There have been many methods such as chemical combination by elementary substances directly (Rundqvist 1966), replacement reaction by solidity (Fjellvag et al. 1984) chemical reaction between metal halide lamp and phosphate (Rowley and Parkin 1993) decomposition of organic compound of metal (Gingerich 1964), electrolysis of molten salt (Li et al. 1998), and deoxidization of phosphate, etc. (Gopalakrishnan et al. 1997) to produce them. But none of the above methods is with the advantages of being clean, low energy consumption and easy-to-approach conditions.
Stephanie L. Brock and her co-workers have firstly reported solvothermal syntheses of Cu3P (Aitken et al. 2005). Thereafter, our group has synthesized micrometer phosphides and nanometer Co2P with hydrothermal method using red phosphorus as raw material (Liu et al. 2010; Huang et al. 2010, 2011). Until now, few related studies have paid any attention to the above-mentioned synthesis process. In the present work, synthesis of nanometer Ni2P and Ni12P5 by hydrothermal process has been presented. The hydrothermal experiments were conducted under a relative low temperature (200°C) for only 10 h with red phosphor as raw material. Important parameters like ratio of raw material and pH of reaction system have been considered to study their effect on powder characteristics such as phase, morphology and particle size. According to the experimental results, a hydrothermal method to synthesize nanometer nickel phosphide compounds (Ni2P and Ni12P5) has been summarized in this paper.
Experiments
Preparation of the reacting suspension and samples
Starting materials used in the experiments were analytical reagents. All the reagents were purchased from Sinopharm Chemical Reagent Co., Ltd and used as received. The red phosphor (P) and NiCl2·6H2O (≥99.0%) were used as phosphor and nickel sources. KOH (≥82.0%) was used to establish an alkali reacting environment. The reacting suspension was prepared as follows: first, red phosphor was ground in a mortar to get well-distributed small powders. Then a desired amount of NiCl2·6H2O and the as-ground red phosphor were added to 64 ml distilled water under vigorous stirring to get mixed aqueous suspension (Table 1). KOH as a variable factor of the process was only used in Groups E, F, G and H.
The prepared suspension was poured into a Teflon-lined autoclave with 0.8 filling factor, sealed, and hydrothermally treated at 200°C for 10 h. After the autoclave cooled to room temperature, the black products were collected and washed with plenty of distilled water. They were then dried at 50°C for 5 h in the air.
Characterization
Phase constitution, chemical composition and morphology of the samples were characterized by X-ray powder diffraction (XRD, Model D/max Rigaku Co., Japan) with Cu Kα radiation (40 kV, 150 mA), energy dispersive X-ray spectroscopy (Oxford Instruments’ INCA EDS system), scanning electron microscopy (SEM, Model JSM-840, JEOL Co., Japan), and transmission electron microscopy (TEM, Model JEM-1200EX, JEOL Co., Japan), respectively.
Results and discussions
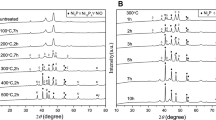
The X-Ray diffraction patterns of as-prepared products are presented in Figs. 1 and 2. The sharp peaks indicated that all the samples were well crystallized. Diffraction peaks of Groups A and B could be readily indexed to Ni12P5 with a small amount of Ni2P. When the starting molar ratios of P/Ni were 25:1 and 30:1 (Groups C and D), respectively, the reflection peaks had a good agreement with the crystalline phase of Ni2P without any impurity.
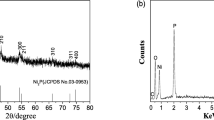
Groups E and F had almost the identical XRD patterns with Group A and Group B, which had a good agreement with the crystalline phase of Ni12P5. The products of Groups C, D, G, and H were all Ni2P. The XRD results were corroborated by the EDS test. The EDS spectra of Groups A and G in Fig. 3 show the presence of Ni and P in the final products, and no impurity peaks were found. The Ni/P ratios in Fig. 3 were 2.29:1 and 2.09:1, respectively.
But enormous differences in size were observed through their SEM and TEM images (Fig. 4). The obtained products with KOH (Groups E–H) had a relatively homogeneous size with an average particle size of about 30 nm, while the sizes of samples synthesized without KOH (Groups A–D) varied from 1 to 100 μm.
The crystal sizes of Groups E, F, G and H were calculated with Scherrer equation (Patterson 1939): Dc = 0.89λ/(B·cosθ), where Dc is the diameter of the particles; λ = 1.518 Å (Cu Ka radiation wavelength); B is the full width at half maxima and θ is the Bragg’s angle. By substituting these values, the size of the nanoparticles was found to be about 10–40 nm, which were in close agreement with TEM results. Images of the samples in Fig. 4 also show that none of the as-prepared powders had uniform and regular shape.
The probable reaction process for the formation of Ni2P/Ni12P5 could be summarized as follows (Wang et al. 2008).
Obviously, KOH in the present study could provide OH− for Eq. 1 and facilitate phosphor to dissolve in water. Companied with the dissolution of phosphor, smaller and relatively homogeneous red phosphor particles were obtained in the suspension. As a result, the reaction between red phosphor and nickel chloride tended to get smaller products. The effect of KOH on the particle size could be observed easily in Fig. 4.
Conclusions
In summary, nanometer nickel phosphide compounds (Ni2P and Ni12P5) were successfully synthesized in a mild hydrothermal process based on the reactions of red phosphor and nickel chloride. Molar ratio of raw materials was the critical factor for the type of final products, and the alkaline environment was necessary to get nanoparticles in the present work.
References
Aitken JA, Ganzha-Hazen V, Brock SL (2005) Solvothermal syntheses of Cu3P via reactions of amorphous red phosphorus with a variety of copper sources. J Solid State Chem 178:970–975
Fjellvag H, Kjekshus A, Andresen AF (1984) Magnetic and structural properties of transition metal substituted MnP. III. Mn1–tFetP (0.00 ≤ t ≤ 0.30). Acta Chem Scand A 38:709–718
Gingerich KA (1964) Vaporization behavior and phosphorus decomposition pressures of tungsten monophosphide. J Phys Chem 68(4):768–772
Gopalakrishnan J, Pandey S, Rangan KK (1997) Convenient route for the synthesis of transition-metal pnictides by direct reduction of phosphate, arsenate, and antimonate precursors. Chem Mater 9(10):2113–2116
Huang X, Liu Z, Dai J, Zhu Z (2010) A hydrothermal method for the synthesis of phosphides. CN Patent 101659403B
Huang H, Huang X, Zhu Z, Dai J (2011) Hydrothermal synthesis of cobalt phosphide nanoparticles. Ceram Int. doi:10.1016/j.ceramint.2011.09.001
Li W, Dhandapani B, Oyama ST (1998) Molybdenum phosphide: a novel catalyst for hydrodenitrogenation. Chem Lett 28(3):207
Liu Z, Huang X, Zhu Z, Dai J (2010) A simple mild hydrothermal route for the synthesis of nickel phosphide powders. Ceram Int 36:1155–1158
Muettcrties EL, Sauer JC (1974) Catalytic properties of metal phosphide. Qualitative assay of catalytic properties. I. J Am Chem Soc 96(11):3410–3415
Oyama ST (2003) Novel catalysts for advanced hydro processing: transition metal phosphides. J Catal 216:343–352
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56(10):978
Rowley AT, Parkin IP (1993) Convenient synthesis of lanthanide and mixed lanthanide phosphides by solid-state routes involving sodium phosphide. J Mater Chem 3(7):689–692
Rundqvist S (1966) New metal-rich phosphides of niobium, tantalum, and tungsten. Nature 211(50–51):847–848
Wang X, Wan F, Gao Y, Liu J, Jiang K (2008) Synthesis of high-quality Ni2P hollow sphere via a template-free surfactant-assisted solvothermal route. J Cryst Growth 310:2569–2573
Acknowledgments
Financial support from Ocean University of China through Young Teachers’ Scientific Research Special Fund Projects (No. 201113038) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wang, B., Huang, X., Zhu, Z. et al. Hydrothermal synthesis method of nickel phosphide nanoparticles. Appl Nanosci 2, 423–427 (2012). https://doi.org/10.1007/s13204-012-0057-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0057-0