Abstract
The effect of hydrogen addition on the catalytic activity and product selectivity of PNP/Cr(III)/MAO catalytic systems for ethylene tetramerization was investigated. The results showed that the catalytic activity could be increased twice and the polymer production could be reduced efficiently through hydrogen addition. It could be inferred from the analysis and characterization results of the products that there existed at least three sorts of catalytic active centers in the ethylene tetramerization reaction.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In 2004, researchers from Sasol reported the first catalyst for the selective conversion of ethylene to 1-octene, which was consisted of PNP ligand, chromium source and methylaluminoxane [1]. Since its discovery, a rapid increase in the number of publications and patents have been reported for selective ethylene tetramerization, among which much attention has been paid to the electronic and steric factors of the PNP ligands, reaction mechanism, reaction kinetics and new ligands [2–5].
Hydrogen has been used as a chain-transfer agent to control the molecular weight of the polymer in the commercial production of polyolefins, such as polypropylene and polyethylene. However, the production of polymer, which leads to both lower yields and reactor fouling, has not been investigated thoroughly. Hydrogen has been invited to reduce the molecular weight of the polymer and enhance the activity to extent in ethylene trimerization reactions catalyzed by chromium-based systems [6–10].
In the case of ethylene tetramerization catalytic systems, to the best of our knowledge, the effect of hydrogen on catalytic performance was investigated only in one paper by Bollmann et al. [1]. It was described that the addition of 2.5 bar of hydrogen to the system resulted in a remarkable decrease in solids formation (PE selectivity from 1.05 to 0.07 %), with little effect on oligomer selectivity and productivity. Lower pressure of hydrogen has not been investigated in detail.
We herein report the result of our research on the effect of hydrogen on the performance of PNP/Cr(III)/MAO catalytic system applied in ethylene tetramerization. More specifically, the low pressure of hydrogen is investigated in detail. It is found that low addition of hydrogen has a remarkable influence on productivity.
Experimental
All manipulations were conducted under nitrogen atmosphere using standard Schlenk techniques or in a glove box.
Materials
The bis(diphenylphosphino)isopropylamine (PNP) ligand was synthesized according to the reported procedures [1]. CrCl3(THF)3 was purchased from Aldrich and used as received. Polymerization grade ethylene, high-purity nitrogen (99.999 %) and high purity hydrogen (99.999 %) were purchased from Tianjin Summit Specialty Gases Co., Ltd. (China) and no further purification was carried out. Methylaluminoxane (MAO) was purchased from Strem Chemicals, Inc. Cyclohexane was stored over activated molecular sieves 4A and extensively dried and distilled in nitrogen from sodium and diphenyl ketone prior to use.
Ethylene tetramerization
All tetramerizations were carried out in a 1.0 L stainless steel autoclave reactor operated in semi-batch mode. Purified cyclohexane was transferred to the reactor under nitrogen atmosphere. Cocatalyst, PNP ligand and CrCl3(THF)3 solution were injected into the reactor using gas-tight syringes. If necessary, the reactor was initially pressurized with hydrogen to act as promoter. Unless otherwise stated, hydrogen was not fed to the reactor at any other time during the reaction. The ethylene tetramerization reaction was started by feeding gaseous monomer on demand to maintain a constant pressure in the reactor. The flow rate of ethylene fed to the reactor was measured with an on-line mass flow meter. Ethylene tetramerization was ceased by rapid depressurization of the reactor followed by quenching with methanol. The catalytic activity was calculated from the weight of the product. Polymeric product was collected by stirring for 90 min in acidified ethanol and rinsed with ethanol and acetone on a glass frit. The polymer was initially dried on air and subsequently in vacuum at 60 °C for 6 h.
Characterization of product
Liquid products were analyzed by GC-FID employing Agilent Technologies 6890H using n-heptane as an internal standard. Chromatographic conditions: HP-1 capillary column, FID detector, N2 carrier gas at 30 mL/min speed, starting temperature 35 °C kept for 10 min, raised to 280 °C with the rate of 10 °C min−1, 280 °C for sample injector, 300 °C for detector, 0.2 μL for the sample quantity. M w, M n and MWD of PE were determined by a Waters Alliance GPCV-2000 equipped with a refractive index detector at 150 °C, using three Polymer Laboratory MIXED-B columns and 1,2,4-trichlorobenzene as solvent. The number-average and weight-average molecular weight (M n and M w, respectively) were determined with a polystyrene standard calibration. The melting temperature and enthalpy of fusion (△H f) of PE were measured on a Perkin-Elmer DSC-7 differential scanning calorimeter in the following manners. First, the sample was heated to 210 at 10 °C/min and maintained the temperature for 5 min to remove the thermal history. Then, it was cooled to 50 at 10 °C/min, followed by reheating at 10 °C/min. The thermogram of each sample was recorded in the second heating run. The catalytic activity was calculated from the weight of the product.
Results and discussion
Effects of H2 partial pressure on catalytic activity and product selectivity
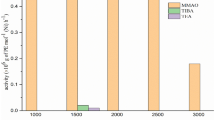
Hydrogen was introduced to ethylene tetramerization over PNP/Cr(III)/MAO either at its start or during the run, and the results are shown in Table 1 and Fig. 1. The presence of hydrogen in the reactor significantly increased the catalyst activity for all runs. 0.03 MPa of hydrogen pressures established in the reactor before the inlet of ethylene can greatly improve the catalytic activities. Increasing hydrogen pressure from 0.03 to 0.10 MPa (Table 1, entry 2–4) did not increase catalyst activity obviously. The selectivity to 1-hexene was increased and the selectivity to 1-octene was decreased slightly with the increase of hydrogen pressure. After the removal of hydrogen from the reactor, the catalytic activity decreased to the values as initially observed (Fig. 1, stage C). By comparing the result of entry 1 to entry 4 in Table 1, it was noticed that the catalytic activity almost doubled by introducing a small amount (0.03 to 0.10 MPa) of hydrogen into the reaction system. With the further increase of hydrogen’s pressure, the catalytic activities decreased (Table 1, entry 5 and 6). For reactions catalyzed by less amount of Cr, the same trend of higher catalytic activity with the increase of hydrogen pressure was observed (Table 1, entry 7 and 8).
Compared the values in Table 1, entry 4 and entry1, there is onefold increase in productivity.
Reaction conditions: reaction temperature: 50 °C; Solvent: cyclohexane; n(Al):n(Cr(III)) = 200:1; c(Cr) = 2.0 μmol L−1. Stage A: ethylene pressure 4.5 MPa, without hydrogen; Stage B: ethylene pressure 4.5 MPa and hydrogen pressure 0.1 MPa; Stage C: the polymerization was interrupted by stopping the monomer flow and depressurizing the reactor. The reactor was then connected to the vacuum line for 1 min to eliminate residual amounts of hydrogen dissolved in the cycolhexane. The tetramerization was restarted by pressurizing the reactor with ethylene pressure 4.5 MPa, this time in absence of hydrogen.
A number of explanations of hydrogen effect on Ziegler–Natta catalyst and metallocene catalyst for olefin polymerization have been proposed, including hydrogen effects on the number of active centers [6, 7], oxidation of Ti2+ to Ti3+ species [11], hydrogenation of inactive Ti allylic species [12, 13] and hydrogenation of unsaturated chain ends that poison active sites. Although progress has been made in understanding the mechanism of PNP/Cr(III)/MAO catalyzed ethylene tetramerization [5, 14], most notably the role of a metallacyclic reaction manifold, definitive answers relating to the oxidation state of chromium and specific aspects of ligand control remain elusive. The plausible machenism of how H2 promotes to reactivate the deactive metal centre is shown in Fig. 2. As reported by Rabeah and co-workers [15], in the case of PNP/Cr catalytic system, a (PNP)Cr(II)(CH3)2 complex stemmed from Cr(III) species is regarded as the active species for selective tetramerization by passing a reversible redox cycle, while reduction to Cr(I) leads to deactivation. According to the above results, we consider that the hydrogen could oxidate the deactive Cr(I) to reactive Cr(III) species in some extent.
Effects of H2 partial pressure on PE formation and molecular weight
In ethylene tetramerization catalyzed by PNP/Cr(III)/MAO, formation of about 0.3–2.0 wt% of polyethylene is unavoidable [1]. The polymer formation not only decreases the yield of target product, but also cause pipeline fouling under operating conditions (50–80 °C, cyclohexane solvent). There are two kinds of polymer with different molecular weight in the product, as shown in Table 2. One is wax polymer, molecular weight of which is below 2000. The wax was proved to be linear α-olefins by 1H NMR. The other is filament polyethylene. Typically, M w lies in the range of 200,000–750,000 g/mol with molecular weight distributions (MWD) of around 3.5. 13C NMR measurement revealed that the filament polyethylene was linear high-density polyethylene. As expected, the average molecular weight of filament polyethylene decreased with increasing hydrogen pressure because of chain transfer effect. However, the molecular weight averages of wax did not change with the change of hydrogen pressure in the reactor. From these results, it can be speculated that wax polyethylene and filament polyethylene were obtained following different reaction mechanism. The PE formation reaction was catalyzed by at least two different species.
For a single-site polymerization catalyst under constant conditions (temperature and ethylene concentration), the MWD would have Flory’s theoretical value of 2. Broader MWDs proves the existence of multiple species. According to the value of polymer, we infer that selective oligomerization product and oligomer wax were obtained through cyclization mechanism, while high-molecular weight PE may be obtained through a linear chain growth alkene insertion mechanism (Cossee mechanism) [16].
Reaction conditions: reaction temperature: 50 °C; reaction pressure: 4.5 MPa; reaction time: 60 min; n(Al):n(Cr(III)) = 200:1; c(Cr) = 2.0 μmol L−1; solvent: cyclohexane.
Conclusions
The effect of hydrogen addition on the catalytic performance of PNP/Cr(III)/MAO catalyst for ethylene tetramerization and the molecular weight of the polymer was investigated. It was proved that the addition of hydrogen may not only significantly increase the catalytic activity, but also decrease the polymer formation, which may solve the fouling problem in industrial operation. It could be inferred from the analysis and characterization results of the products that there existed at least three sorts of catalytic active centers in the ethylene tetramerization reaction.
References
Bollmann A, Blann K, Dixon JT, Hess FM, Killian E, Maumela H, McGuinness DS, Morgan DH, Neveling A, Otto S (2004) Ethylene tetramerization: a new route to produce 1-octene in exceptionally high selectivities. J Am Chem Soc 126:14712–14713
Van Leeuwen PW, Clément ND, Tschan MJ-L (2011) New processes for the selective production of 1-octene. Coord Chem Rev 255:1499–1517
Agapie T (2011) Selective ethylene oligomerization: recent advances in chromium catalysis and mechanistic investigations. Coord Chem Rev 255:861–880
Belov G (2012) Tetramerization of ethylene to octene-1 (a review). Pet Chem 52:139–154
Britovsek GJ, McGuinness DS, Wierenga TS, Young CT (2015) Single and double coordination mechanism in Ethylene Tri- and Tetramerization with Cr/PNP catalysts. ACS Catalysis 5(7):4152–4166
Murtuza S, Harkins SB, Long GS, Sen A (2000) Tantalum- and titanium-based catalytic systems for the synthesis of hyperbranched polyethene. J Am Chem Soc 122:1867–1872
Hagen H, Kretschmer WP, van Buren FR, Hessen B, van Oeffelen DA (2006) Selective ethylene trimerization: a study into the mechanism and the reduction of PE formation. J Mol Catal A Chem 248:237–247
Sydora OL, Jones TC, Small BL, Nett AJ, Fischer AA, Carney MJ (2012) Selective ethylene tri-/tetramerization catalysts. ACS Catalysis 2:2452–2455
Goode MG, Spriggs TE, Levine IJ, Wilder WR, Edwards CL, in, Google Patents, 1992
Parsons I, Al-Turki T (1989) On the mechanism of action of hydrogen added to propene polymerizations using supported titanium chloride catalysts with a phthalate ester/silane stereoregulating donor pair. Polymer Commun 30:72–73
Kioka M, Kashiwa N (1991) Study of the activity enhancement caused by the addition of hydrogen in olefin polymerization. J Macromol Sci–Chem 28(9):865–873
Bukatov GD, Goncharov VS, Zakharov VA (1995) Number of active centers and propagation rate constants in the propene polymerization on supported Ti–Mg catalysts in the presence of hydrogen. Macromol Chem Phys 196:1751–1759
Overett MJ, Blann K, Bollmann A, Dixon JT, Haasbroek D, Killian E, Maumela H, McGuinness DS, Morgan DH (2005) Mechanistic investigations of the ethylene tetramerisation reaction. J Am Chem Soc 127:10723–10730
Rabeah J, Bauer M, Baumann W, McConnell AE, Gabrielli WF, Webb PB, Selent D, Brückner A (2012) Formation, operation and deactivation of Cr catalysts in ethylene tetramerization directly assessed by operando EPR and XAS. ACS Catalysis 3:95–102
Cossee P (1964) Ziegler-Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts. J Catal 3:80–88
Acknowledgments
This work was greatly supported by the National Natural Science Foundation of China (51203115).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jiang, T., Zhang, L., Gao, J. et al. Hydrogen: efficient promoter for PNP/Cr(III)/MAO catalyzed ethylene tetramerization toward 1-octene. Appl Petrochem Res 6, 413–417 (2016). https://doi.org/10.1007/s13203-016-0151-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-016-0151-4