Abstract
In this study, we examined catalyst systems comprising chromium(III) chloride tetrahydrofuran, diphenylphosphinoamine (PNP) ligand, and a mixed activator of methylaluminoxane (MAO) and alkylaluminum for the selective tetramerization of ethylene. By comparing the catalytic activities and the selectivities toward 1-hexene and 1-octene, we investigated the effects of various mixed aluminoxane systems on the ethylene tetramerization process. MAO/trimethylaluminum, MAO/triethylaluminum, and MAO/triisobutylaluminum were all effective cocatalysts for the PNP/Cr(III) ethylene tetramerization system, providing high catalytic activities and high selectivities. When using MAO/diethylaluminumchloride as the cocatalyst, PNP/Cr(III) exhibited an impressive switch in selectivity: from ethylene tetramerization to ethylene trimerization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1-Hexene and 1-octene are versatile intermediates for petrochemical industry and widely used as co-monomers for the production of linear low-density polyethylene [1]. Recent years have witnessed considerable activity in the tri- and tetramerization of ethylene for the production of 1-hexene and 1-octene, respectively [2–4]. These routes largely avoid the production of undesirable olefins that conventional full-range oligomerization processes produce. Since the first report of ethylene tetramerization toward 1-octene using chromium/bis(diphenylphosphino)amine (PNP) catalysts [5], many studies have been made into the reaction mechanism and kinetics, the structure and properties of the ligand, and the effects of cocatalysts [6–16]. The ethylene tetramerization process typically requires a high methylaluminoxane (MAO) chromium molar ratio (ca. 300), which is undesirable because of the high cost of MAO. Considerable research has been undertaken to afford alternative cocatalysts exhibiting catalytic productivity and selectivity similar as those of MAO. McGuinness [17] developed well-defined, stoichiometric cocatalysts for ethylene tetramerization from studies of a number of PNP/Cr(III) catalysts activated using B(C6F5)3/AlR3 or [Ph3C][B(C6F5)4]/AlR3. Although the liquid fraction selectivities were similar to that obtained with MAO activation, these catalysts deactivate rapidly and provided variable amounts of polyethylene formation.
It is well known for metallocene and constrained geometry catalysts, that the additives of TIBA can increase cocatalytic activity of MAO, and prolong the catalyst life [18–21]. In this present study, we employed several less-expensive aluminum alkyls and a small amount of MAO to construct mixed aluminoxane systems and then explored their influence on ethylene oligomerization based on the PNP/Cr(III) catalyst system.
Experimental
All manipulations were conducted under an N2 atmosphere using standard Schlenk techniques or in a glove box.
Materials
PNP ligand was synthesized according to a published method [15]. CrCl3(THF)3 was purchased from Aldrich and used as received. Polymerization-grade ethylene was obtained from Tianjin Saimeite Specialty Gases (China). Trimethylaluminum (TMA, 98 %), triethylaluminum (TEA, 93 %), tri-n-hexylaluminum (TNHA, 95 %), diethylaluminum chloride (DEAC, 97 %), triisobutylaluminum (TIBA, 1.1 mol/L in toluene), ethylaluminum dichloride (EADC, 1.0 mol/L in hexane), and methylaluminoxane (MAO, 1.4 mol/L in toluene) were purchased from Aldrich and used as received. Cyclohexane was stored over activated 4-Å sieves; it was dried and distilled under N2 from sodium/diphenylketone prior to use.
Oligomerization of ethylene
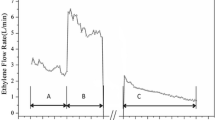
Ethylene oligomerization was performed in a transparent glass reaction vessel, equipped with a pressure meter and needle valves for sample injections, with magnetic stirring and heating in an oil bath. After evacuation and flushing with N2 (three times) and ethylene (twice), the reaction vessel was charged with a quantity of cyclohexane and magnetically stirred under ambient ethylene atmosphere. When the desired reaction temperature was established, a quantity of MAO/alkylaluminum activator, PNP ligand and CrCl3(THF)3 were injected into the reactor. Typically 30 min later, the mixture was rapidly cooled to 0 °C and then the reaction was quenched through the addition of EtOH/HCl (10 wt%). The catalytic activity was calculated from the increase in product weight.
Characterization of product
The distribution of the oligomers in the resulting solution was determined through gas chromatographic analysis using an Agilent Technologies 7890A instrument equipped with a 30 m × 0.25 mm column and a flame ionization detector. The solution was analyzed using a temperature program—from 35 to 280 °C (10 °C/min, hold 10 min)—with n-heptane as the internal standard. Product selectivities were calculated using gas chromatography/mass spectrometry (GC/MS) to analyze the collected gases and obtained solutions.
Results and discussion
In Sasol’s initial report [5], the optimal results when using the PNP/Cr(III)/MAO catalyst system for ethylene tetramerization were obtained at an MAO-to-Cr molar ratio of 300. To examine the effect of the added alkylaluminum on the catalytic system, in this study we kept the MAO/Cr ratio constant at 100 in all runs. To test the effects of the cocatalysts, we mixed an AlR3 species (TMA, TEA, TIBA, TNHA, DEAC, or EADC) with MAO at various molar ratios and then measured the resulting ethylene tetramerization activities and product selectivities.
Effect of MAO/TMA mixtures on catalytic activity and product selectivity
Table 1 lists the effect of the TMA/Cr molar ratio on the catalytic activity and product selectivity provided by PNP/Cr(III)/MAO/TMA catalytic systems. In general, the addition of TMA to MAO decreased the catalytic activity. A threshold appears to exist for the amount of TMA added to MAO that is necessary to produce an effective cocatalyst. One reason could be that TMA controls the Lewis acidity of MAO through coordination to its oxygen atoms; an excess of TMA may, therefore, interfere with the formation of active Cr species and/or lead to over-reduction of the Cr species. The total selectivity toward 1-octene and 1-hexene remained constant at 94 % regardless of the Al/Cr molar ratio over the tested range of TMA contents.
Effect of MAO/TEA mixtures on catalytic activity and product selectivity
Table 2 reveals that TEA affected the 1-octene selectivity and catalytic activity in PNP/Cr(III)/MAO/TEA catalytic systems. Upon increasing the TEA/Cr molar ratio from 0 to 10, the catalytic activity and the selectivity toward 1-octene both increased. When the TEA/Cr molar ratio was 10, the catalytic activity and the selectivity toward 1-octene were higher than the optimal results for the tetramerization of ethylene using the PNP/Cr(III)/MAO catalyst system at an MAO/Cr ratio of 300. Interestingly, an excess of TEA in the mixed aluminoxane system decreased the catalytic activity and increased the selectivity toward 1-butene (runs 13–17). Thus, the mixed aluminoxane derived from MAO and TEA was a decent cocatalyst for the oligomerization of ethylene based on the PNP/Cr(III) system.
Effect of MAO and TIBA mixtures on catalytic activity and product selectivity
Table 3 presents the efficacy of MAO and TIBA mixtures as cocatalysts in the catalytic system for ethylene tetramerization. The mixed aluminoxane formed from MAO/TIBA provided the PNP/Cr(III) catalyst system with very high catalytic activity, relative to that of standard MAO activation under the same conditions. The catalytic activity increased initially upon increasing the molar ratio of TIBA to Al/Cr, reaching a maximum at a ratio of approximately 50 (run 19). The activity under the best conditions (run 19) was higher than that obtained at a molar ratio of MAO to Al/Cr of approximately 100 (run 2) and almost equal to that obtained when the MAO to Al/Cr molar ratio was approximately 300 (run 1). In addition, the introduction of MAO/TIBA as mixed aluminoxanes resulted in almost no change in product selectivity. With this mixed aluminoxane system, we could decrease the amount of MAO and, thereby, lower the cost of the process without changing the product distribution of ethylene oligomerization when using the PNP/Cr(III) catalyst system.
Effect of MAO and TNHA mixtures on catalytic activity and product selectivity
Table 4 displays the results for ethylene tetramerizations performed at various molar ratios of TNHA to Al/Cr. Upon increasing this molar ratio from 10 to 200, we observed sharp decreases in the catalytic activity and selectivity toward 1-octene and 1-hexene and a substantial increase in the selectivity toward 1-butylene. The poor performance of these mixed MAO/TNHA aluminoxanes was presumably due to the large bulk of the alkyl groups in TNHA inhibiting concerted catalysis. In general, methylcyclopentane and methylenecyclopentane are formed in a ratio of 1:1 and are the third most abundant products, after 1-octene and 1-hexene, of ethylene tetramerization in PNP/Cr(III)/MAO catalytic systems. Unexpectedly, we found that the selectivity toward methylcyclopentane increased significantly upon increasing the molar ratio of TNHA to Al/Cr from 10 to 200, with a conspicuous decrease in the amount of methylenecyclopentane in the product.
These observations suggest the hexenyl/hydride-mediated pathway. The n-hexyl units of TNHA are strong electron-donating groups that decrease the amount of positive charge at the Cr metal center—a situation that is conducive to the generation of methylcyclopentane rather than methylenecyclopentane (Scheme 1).
Effect of MAO and DEAC mixtures on catalytic activity and product selectivity
The catalytic activity and product selectivity when using MAO/DEAC as a mixed aluminoxane in ethylene oligomerization were affected strongly by the molar ratio of DEAC to Al/Cr. Table 5 reveals that the catalytic activity increased upon increasing the molar ratio of DEAC to Al/Cr, reaching a maximum at a value of 200, but decreased thereafter. Although the 1-octene selectivity was extremely low, we obtained satisfactory activity and selectivity toward 1-hexene with this mixed aluminoxane when the DEAC/Cr ratio was 200. This system, employing MAO/DEAC as the cocatalyst, exhibited an impressive switch in selectivity of the PNP/Cr(III) catalyst from ethylene tetramerization to ethylene trimerization. This cocatalyst had another interesting effect on the catalytic system because it increased the selectivity toward methylcyclopentane when we increased the molar ratio of DEAC to Al/Cr from 10 to 500. We found, however, that the selectivity toward methylenecyclopentane decreased when using DEAC as a cocatalyst. The formation of methylcyclopentane and methylenecyclopentane presumably occurs through a disproportionation process.
Effect of MAO and EADC mixtures on catalytic activity and product selectivity
Table 6 presents the results for the ethylene oligomerizations performed using the mixed aluminoxane MAO/EADC as the cocatalyst. In the presence of EADC, ethylene oligomerization occurred with low catalytic activity relative to that of the standard MAO activation process. We observed an increase in the yield of 1-hexene relative to 1-octene and, simultaneously, an obvious increase in the yield of methylcyclopentane upon increasing the molar ratio of EADC to Al/Cr, similar to DEAC system; we attribute this performance to the presence of the chlorine atoms in these two aluminum alkyls.
The added trialkylaluminum compound could interact with the active species in the following two ways: one is the interaction with the counter anion, and the other is the interaction with the coordinatively unsaturated cationic chromium species to form a heterobinuclear complex. The use of excess MAO has economic implications when it comes to the large-scale use in industry. TMA, TEA and TIBA were the most important and functioned as both reducing agents and cocatalysts. They were readily available and relatively inexpensive and performed well with Cr(III)/PNP/MAO for ethylene tetramerization toward 1-octene.
Conclusions
To decrease the cost of the cocatalyst, herein we tested the effects of six relatively inexpensive aluminum alkyls (TMA, TEA, TIBA, TNHA, DEAC, EADC) to partially replace MAO in the PNP/Cr(III)/MAO catalyst system for ethylene oligomerization. We achieved satisfactory catalytic activity and product selectivity when using a system of mixed aluminoxane activators, comprising MAO and various aluminum alkyls as cocatalysts. Amazingly, the application of MAO/DEAC as the cocatalyst resulted in an impressive switch in the selectivity of the PNP/Cr(III) catalytic system—from ethylene tetramerization to ethylene trimerization—as well as high activity. Further research into other mixed aluminoxane systems and their applications in ethylene oligomerization are ongoing.
References
Sun WH, Zhang W, Gao TL, Tang XB, Chen LY, Li Y, Jin XL (2004) Synthesis and characterization of N-(2-pyridyl) benzamide-based nickel complexes and their activity for ethylene oligomerization. J Organomet Chem 689:917–929
Dixon JT, Green MJ, Hess FM, Morgan DH (2004) Advances in selective ethylene trimerisation—a critical overview. J Organomet Chem 689:3641–3668
Piet WNM, Nicolas D, Mathieu JLT (2011) New processes for the selective production of 1-octene. Coord Chem Rev 255:1499–1517
Agapie T (2011) Selective ethylene oligomerization: recent advances in chromium catalysis and mechanistic investigations. Coord Chem Rev 255:861–880
Bollmann A, Blann K, Dixon JT, Hess FM, Killian E, Maumela H, McGuinness DS, Morgan DH, Neveling A, Otto S, Overett MJ, Slawin AMZ, Wasserscheid P, Kuhlmann S (2004) Ethylene tetramerization: a new route to produce 1-octene in exceptionally high selectivities. J Am Chem Soc 126:14712–14713
Blann K, Bollmann A, Dixon JT, Hess FM, Killian E, Maumela H, Morgan DH, Neveling A, Otto S, Overett MJ (2005) Highly selective chromiumbased ethylene trimerisation catalysts with bulky diphosphinoamine ligands. Chem Commun 620–621
Overett MJ, Blann K, Bollmann A, Dixon JT, Hess F, Killian E, Maumela H, Morgan DH, Neveling A, Otto S (2005) Ethylene trimerisation and tetramerisation catalysts with polar-substituted diphosphinoamine ligands. Chem Commun 622–624
Blann K, Bollmann A, Bod H, Dixon JT, Killian E, Nongodlwana P, Maumela MC, Maumela H, McConnell AE, Morgan DH, Overett MJ, Prétorius M, Kuhlmann S, Wasserscheid P (2007) Ethylene tetramerisation: subtle effects exhibited by N-substituted diphosphinoamine ligands. J Catal 249:244–249
Kuhlmann S, Blann K, Bollmann A, Dixon JT, Killian E, Maumela MC, Maumela H, Morgan DH, Prétorius M, Taccardi N, Wasserscheid P (2007) N-substituted diphosphinoamines: toward rational ligand design for the efficient tetramerization of ethylene. J Catal 245:279–284
Elowe PR, McCann C, Pringle PG, Spitzmesser SK, Bercaw JE (2006) Nitrogen-linked diphosphine ligands with ethers attached to nitrogen for chromium-catalyzed ethylene tri-and tetramerizations. Organometallics 25:5255–5260
Weng ZQ, Teo SH, Hor TSA (2007) Chromium(III) catalysed ethylene tetramerization promoted by bis(phosphino)amines with an N-functionalized pendant. Dalton Trans 3493–3498
Killian E, Blann K, Bollmann A, Dixon JT, Kuhlmann S, Maumela MC, Maumela H, Morgan DH, Nongodlwana P, Overett MJ, Prétorius M, Höfener K, Wasserscheid P (2007) The use of bis(diphenylphosphino) amines with N-aryl functionalities in selective ethylene tri-and tetramerisation. J Mol Catal A Chem 270:214–218
Jiang T, Zhang S, Jiang XL, Yang CF, Niu B, Ning YN (2008) The effect of N-aryl bisphosphineamine ligands on the selective ethylene tetramerization. J Mol Catal A Chem 279:90–93
Jiang T, Chen HX, Ning YN, Chen W (2006) Preparation of 1-octene by ethylene tetramerization with high selectivity. Chin Sci Bull 51:521–523
Mao GL, Ning YN, Hu WB, Li SM, Song XF, Niu B, Jiang T (2008) Synthesis of a novel triple-site diphosphinoamine (PNP) ligand and its applications in ethylene tetramerization. Chin Sci Bull 53:3511–3515
Kuhlmann S, Dixon JT, Haumann M, Dixon JT, Ofili J, Spuhl O, Taccardi N (2006) Influence of elevated temperature and pressure on the chromium-catalysed tetramerisation of ethylene. Adv Synth Catal 348:1200–1206
McGuinness DS, Overett M, Tooze RP, Blann K, Dixon JT, Slawin AMZ (2007) Ethylene tri- and tetramerization with borate cocatalysts: effects on activity, selectivity, and catalyst degradation pathways. Organometallics 26:1108–1111
Ioku A, Hasan T, Shiono T, Ikeda T (2002) Effects of cocatalysts on propene polymerization with [t-BuNSiMe2(C5Me4)]TiMe2. Macromol Chem Phys 203:748–755
Shiono T, Yoshida S, Hagihara H, Ikeda T (2000) Additive effects of trialkylaluminum on propene polymerization with (t-BuNSiMe2Flu)TiMe2-based catalysts. Appl Catal A-Gen 200:145–152
Kleinschmidt R, Leek YVD, Reffke M, Fink G (1999) Kinetics and mechanistic insight into propylene polymerization with different metallocenes and various aluminium alkyls as cocatalysts. J Mol Catal A Chem 148:29–41
Bhriain NN, Brintzinger HH, Ruchatz D, Fink G (2005) Polymeryl exchange between ansa-zirconocene catalysts for norbornene-ethene copolymerization and aluminum or zinc alkyls. Macromolecules 38:2056–2063
Acknowledgments
This study was supported by the National Natural Science Foundation of China (U1162114), Program for New Century Excellent Talents in University (NCET-07-0142) and the Provincial Key Laboratory of Oil & Gas Chemical Technology (HXHG2012-04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Wang, T., Gao, X., Shi, P. et al. Mixed aluminoxanes: efficient cocatalysts for bisphosphineamine/Cr(III) catalyzed ethylene tetramerization toward 1-octene. Appl Petrochem Res 5, 143–149 (2015). https://doi.org/10.1007/s13203-015-0103-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-015-0103-4