Abstract
Compounds with acidic and basic functional groups (aspartic acid, arginine, and cystine) are evaluated for inhibition of aldol condensation and dissolution of product in alkaline media. Inhibition efficiency of amino acids were studied at varied mole ratios of acetaldehyde to antipolymerant (2, 5, and 10), reaction temperature, and caustic concentration as a function of time (0.25, 1.0, 2.0 and 24.0 h) using UV spectrophotometry. Results obtained have been compared with efficiency of known amine derivatives. Arginine showed better efficiency when compared with aspartic acid and cysteine. Furthermore, data indicate that presence of carboxylic group in addition to amine group facilitates inhibition as well as dissolution of aldol polymer in alkaline media, while compounds with amine group acts as inhibitor for aldol condensation only.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olefins are produced by naphtha or gas (ethane/propane/other alkane) cracking in petrochemical industry. Olefins are then used as raw material to produce commodity or specialty chemicals and polymers such polyethylene, polypropylene, polyvinyl chloride, mono-ethylene glycol, etc. These chemicals and polymers are used in different sectors such as agriculture, automobile, packaging, life appliances, apparels, etc.
Cracking of naphtha to produce olefins is a highly complex chemical phenomena because it involves multiple reactions of intermediates, reactants, and products. Acid gases such as H2S, CS2, –RSH, etc., are also produced along with olefins. The removal of acid gases is essential for better performance of downstream catalytic purifications units and production of high-purity chemicals and monomers for further conversion to the required chemicals or polymers. The process for removal of acid gases in naphtha cracker involves treatment of cracked product with caustic solution in absorption column. This facilitates the removal of acid gases from other chemicals in the form of Na2S. The acetaldehyde formed during cracking undergoes aldol condensation in a caustic absorption column, resulting in the formation of polymeric materials or red oil chocking the absorption column. Different types of antipolymerants are therefore added in the absorption column to prevent the formation of polymeric material for increasing the efficiency of acid removal process in naphtha cracking. In view of this, understanding the aldol condensation process of aldehyde and its prevention in alkaline media is critical in naphtha cracking process.
Aldol condensation involving aldehyde group preset in naturally occurring or synthetic chemical [9, 11] is one of the most thoroughly studied reaction in organic chemistry. Aldol condensation is catalyzed by acids as well as bases. Literature studies indicate that the nature of aldehyde compounds and catalyst type has been investigated in detail for in-depth understanding of condensation process [1, 10, 14, 17, 18, 21]. However, no studies are reported in published literature except patents for prevention of aldol condensation in alkaline media which is very critical in naphtha cracking process for efficiency improvement.
Patent literature on prevention of aldol condensation in alkaline media indicates amine containing chemicals (primary, secondary amines) and borohydrates are used as antipolymerants. The amine compounds react with aldehyde to produce imines that prevent aldol polymerization condensation reaction. The electron donating characteristics of antipolymerant [6–8] is an important parameter for determining its efficiency. The examples include hydroxylamine and its salts [15], hydrazine and its derivative [8], carbohydrazine and its derivatives [6], amino phenol, amino benzoic acid and its salts [7], urea [20], ethylenediamine [2], acetoacetate ester compounds [16] and borohydride [4]. In addition to the action of antipolymerant, the mixing phenomena and design of caustic absorption column in naphtha cracker plant are critical in preventing the formation of polymeric material. Subramaniyam et al. [19] explored the concept of using chemicals which not only prevent the formation of polymer, but also help in dissolving the polymeric material if any formed. Examples of such compounds containing both amine and carboxylic acid groups are 2-aminoethanesulfonic acid, 6-amino hexanoic acid, etc.
We have initiated a systematic work on developing high performing chemicals for prevention of aldol condensation in alkaline media and understanding the chemical structure–performance relationship [12, 13]. In the present communication, we report our studies on amino acids (aspartic acid, arginine, and cysteine, Fig. 1) as antipolymerants for the prevention of aldol condensation and dissolution of polymeric material in alkaline media. The results on amino acids are also compared with known compounds such as ethanolamine, hydroxylamine hydrochloride, hydrazine (Fig. 1).
Experimental
Chemicals
Acetaldehyde, aspartic, arginine, and cystine (AR grade, Sigma Aldrich, India.), ethanolamine, hydroxylamine hydrochloride, hydrazine (Labort, India), caustic (AR grade, SD Fine Chemical Product, India) were used for synthesis and performance reactions. Both arginine and aspartic acid are levorotary type isomers. These are naturally occurring amino acids derived from biological or animal sources.
Performance test of antipolymerants for inhibition of aldol reaction
Amino acid (0.018 mol) was mixed with 40 ml (2.5 M) aqueous sodium hydroxide in a flat-bottomed flask and placed on a hot plate at 350 rpm. Acetaldehyde was added under stirring at 350 rpm. The reaction was monitored by measuring transmittance of solution by UV spectrophotometer (PE Lambda35) at 800 nm at different time intervals for inhibition efficiency measurement [20]. Comparative experiment was conducted and UV transmittance was measured by same procedure without addition of antipolymerant and for amine derivatives reported in literature. Effect of amino acid concentration at 2, 5, and 10 mole ratio of acetaldehyde to antipolymerant and effect of temperature at 25, 40, and 55 °C were also investigated.
Performance test of antipolymerants for dissolution of aldol condensation polymer
Acetaldehyde (0.036 mol) was added to 40 ml (2.5 M) caustic solution in 100-ml conical flask under stirring. Color of the solution changes to hazy yellow after few minutes due to aldol condensation of acetaldehyde under alkaline condition. Yellow precipitate of aldol polymer started appearing after 15 min. Amino acid compound (0.018 mol) was added to this solution and transmittance of solution was measured by UV spectrophotometer at time intervals of 0.25, 1, and 2 h at 800 nm to measure dissolution efficiency of amino acid. Performance of the reported amine derivatives was also studied for dissolution of aldol condensation polymer for comparison.
Product characterization of aldol condensation polymer
IR spectra of dried acetaldehyde–amino acid complex were recorded on a Perkin Elmer make Fourier transform infrared spectrometer (FTIR) with 2 cm−1 resolution and 32 scans by mixing the sample with KBr.
Results and discussion
Aldol condensation reactions involving aldehyde groups are discussed in both academia and industry. Mechanism of liquid red oil formation, which is a product of aldol condensation of few numbers of aldehyde monomer, is shown in Scheme 1. Further polymerization leads to the formation of solid polymer.
In base-catalyzed aldol condensation reaction, acidic hydrogen or β-hydrogen of acetaldehyde is eliminated in the presence of hydroxyl ion. Reactive enolate of aldehyde is formed during the reaction which acts as nucleophile and attacks the electrophilic carbon of another carbonyl (aldehyde) molecule forming 2-butenal [3]. It further gives 2,4-hexenal with double bond called red oil (Scheme 1a) which is partially soluble in caustic solution and insoluble in water. Aldol condensation of dissolved oxygenated hydrocarbons (carbonyls, such as aldehyde and ketones) produces polymeric products that are commonly referred to as red oil in caustic tower [5] which further polymerize to give high molecular weight red polymer (Scheme 1b).
Studies on efficiency of antipolymerant for inhibiting aldol condensation reaction
Effect of reaction time
Effect of amino acid compounds (aspartic acid, arginine, and cystine) on inhibition of aldol condensation reaction was investigated at various mole ratios (acetaldehyde/antipolymerant mole ratio of 2.0, 5.0, and 10.0) at 25 °C in 2.5 M caustic solutions as function of time (0.25, 1, 2, and 24 h) as shown in Table 1.
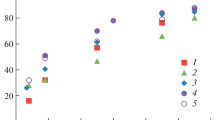
Addition reaction of aldehyde takes place at faster rate when amino acid compounds are not used. The solution turned hazy light yellow to reddish yellow due to the formation of polymer precipitates. Transmittance of the solution was reduced to below 0.1 % after 2 h of reaction (Fig. 2a). The solution turned to a thick slurry in 24 h which can affect the caustic tower efficiency. (Fig. 2b). Arginine indicated 98, 85, and 61 % transmittance at aldehyde to arginine mole ratio of 2, 5, and 10 after 24 h. Transmittance values for cystine were 63, 42, and 14 % and 11, 3 and 0.1 % transmittance for aspartic acid at aldehyde to inhibiting compound mole ratio of 2, 5, and 10 after 24 h.
Presence of amino acid compounds reduced the reaction rate of acetaldehyde to varied efficiency levels depending on their chemical structures. This study as function of time and quantity of such compounds indicated that arginine (among three compounds) inhibits reactions most efficiently followed by cystine and aspartic acid due to presence of two basic group (–NH2) and one acidic group (>COOH). Visually solution remains transparent even after 1–2 h of reaction time (Fig. 2c).
Aspartic acid has shown lowest efficiency probably due to presence of two acid groups (>COOH) at chain end and one basic (–NH2) group making such compound less efficient for inhibiting the reaction. Cystine exhibited better inhibition effect than aspartic acid and less than arginine due to the presence of two basic (–SH and –NH2) functional groups. Perhaps proton associated with –SH has less donating effect compared to –NH2 resulting in lower efficiency than arginine which has two –NH2 groups.
Three reported amine derivatives (ethanolamine, hydroxylamine hydrochloride, hydrazine) were also evaluated for inhibition efficiency. Ethanolamine showed transmittance of 82, 79, and 61 at 2, 5, and 10 mole ratio of acetaldehyde to ethanolamine. Hydroxylamine hydrochloride and hydrazine were also studied under similar condition. Performance of all evaluated compounds showed performance sequence of arginine > ethanolamine > cystine > hydroxylamine hydrochloride > hydrazine > aspartic acid.
It was evident that as ratio of acetaldehyde to inhibitor increased, transmittance of the reaction mixture decreased. Transmittance decreased rapidly for all compounds at molar ratio of 10 which indicated that sufficient inhibitor concentration is required for inhibition of aldol condensation reactions.
During aldol inhibition reaction mechanism, amine group attacks the carbonyl group of acetaldehyde to form carbinolamine intermediate which undergoes loss of water molecule to produce imine [22, 23]. Inhibition reaction is explained in Scheme 2. Imine formation in the case of aspartic acid is quite difficult due to two acidic groups at both the side ends. While in the case of arginine and cystine, electro-donating group present on both ends may be the reason for higher inhibition activity.
Effect of reaction temperature
Effect of reaction temperature on inhibition reaction was investigated at 25, 40, and 55 °C (Table 2) at acetaldehyde to inhibitor mole ratio of 2. Higher temperature increased the rate of reaction as indicated by the decrease in transmittance of solution. This explained that acetaldehydes were converted to polymeric product at faster rate at 55 and 40 °C compared to 25 °C. Results indicated that arginine was most effective inhibitor compared to cystine and aspartic acid. Arginine shows 99 %T at all temperatures, whereas aspartic and cystine show 0.1 and 10 %T, respectively, at 55 °C. Efficiency of inhibitor was in order of arginine > cystine > aspartic acid.
Effect of catalyst concentration
Rate of aldol condensation reaction depends on the presence of catalyst (caustic) concentration; as catalyst concentration increases reaction rate increases. Reducing concentration of the catalyst from 2.5 to 0.625 M exhibited distinct effects on transmittance (Table 3). Reduction in catalyst concentration showed increased transmittance indicating reduction in the formation of aldol polymer. Arginine shows 98–99 % transmittance at all catalyst concentrations after 24 h indicating most effective inhibition efficiency. Cystine is less effective compared to arginine with 63 and 77 % transmittance at 2.5–1.25 M concentrations. Aspartic acid showed lowest efficiency at 11 and 72 % transmittance at 2.5 and 1.25 M concentrations.
FTIR spectroscopy
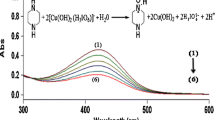
Complexes of acetaldehyde with amino acids were studied through FTIR. Complexes were obtained after evaporation of water on mixing these chemicals in caustic media. Stretching band at 1,650, 1,655, and 1,659 cm−1 in cystine, arginine, and aspartic acid indicated the presence of >C=O of amino acid. Slightly shifted band in all three complexes at 1,584, 1,580, and 1,574 cm−1 (Fig. 3 a, b, c for cystine, aspartic acid, and arginine complexes with aldehydes) indicated >C=N–R (imine) formation between amino acid and acetaldehyde. Shifting of the band revealed formation of strong bond between aldehyde and primary amine group. In all three products, broad band at 3,430, 3,361, and 3,376 cm−1 indicate –OH group of amino acid (Fig. 3a–c). Findings substantiated the theory that acetaldehyde is converted to imine due to the addition of amine-containing antipolymerant in the caustic tower.
Studies on efficiency of compound for dissolution behavior
Effect of amino acid compounds for dissolution of aldol condensation polymer was studied as function of time at aldehyde to amino acid mole ratio of 2 using the methodology described in “Experimental”. Transmittance of solution which did not have amino acid compounds showed <0.1 %T indicating the formation of aldol polymer. When these compounds are added after formation of aldol polymer in 15 min, results indicated that arginine has better efficiency for dissolution of polymer (7–21 %T) compared to aspartic and cystine due to the presence of acidic and basic functional groups (Table 4). Ethanolamine, hydroxylamine hydrochloride, and hydrazine were studied for dissolution of aldol condensation polymer. These compounds showed poor performance compared to arginine, cysteine, and aspartic acid. This suggested that amine group in the compound was not effective for dissolution of aldol condensation polymer. Combination of acidic group (>COOH) and amine group is essential for dissolution of aldol condensation polymer.
Conclusions
Different amino acids were investigated as antipolymerants for inhibition and dissolution reactions of aldehyde formed during olefin production in industrial naphtha cracking process. Effects of antipolymerant concentration, catalyst base concentration, and reaction temperature were investigated as a function of time. Performance of antipolymerants was measured by UV spectrometer. Among the studied compounds, arginine was found to be very effective for inhibition and dissolution reactions compared to aspartic acid and cystine. This study revealed that compounds having higher basicity (electron donating groups) are efficient for inhibition of aldol reaction, but less effective for dissolution purpose. Similarly, more acidic functionality of compound is less effective for inhibition. Study of only amine-containing derivatives for inhibition and dissolution performance indicated the need for both acidic and basic groups in the compound used for the dissolution of aldol condensation polymer.
References
Annunziata R, Cinquini M, Cozzi F, Cozzi PG, Consolandi E (1992) Stereocontrol in the Mukaiyama aldol addition to α-chiral and β-thio-substituted aldehydes. J Org Chem 57:456
Awbrey SS (1990) Method of inhibiting fouling in caustic scrubber systems. US Patent 4952301
Carey FA, Chapter 18: Enols and enolates, http://www.mhhe.com/physscichemistry/carey5e/Ch18/ch18-3-4.html, 30 March 2013
Gary P (1996), Borohydrides to inhibit polymer formation in petrochemical caustic scrubbers. US Patent 5582808
Kurukchi SA, Gondolfe JM (1999) Spent caustic (pre)treatment process. US Patent 5885422
Lewis VE (1992) Method of inhibiting formation of fouling materials during basic washing of hydrocarbons contaminated with oxygen compounds. US Patent 5160425
Lewis VE, Patel NR (1998) Caustic tower trap for acetaldehyde. US Patent 5714055
Lewis VE, Rowe CT (1994) Process for the prevention of polymer formation in compressor systems. US Patent 5288394
Mukaiyama T (1982) The directed aldol reaction. Org React 28:203
Papa AJ (1985) Aldol condensation of enolizable aldehydes using a metal carboxylate catalyst. US Patent 4528405
Paterson I (1988) New symmetric aldol methodology using boron enolate. Chem Ind 12:390
Patil H, Bhajiwala H, Gupta V (2011) A method of cracking petrochemicals. Indian Patent 1706/MUM/2011
Patil H, Bhajiwala H, Gupta V (2011) Energy efficient process for synthesis of salt of aliphatic amino acid. Indian Patent 293/MUM/2012
Radha KP, Lakshmi PY, Munagala A (2011) Benzoic acid catalyzed aldol-type condensation of aldehydes with ethyl diazoacetate: highly diastereoselective. Eur J Org Chem 2011(26):5089–5095
Roling PV (1987) Method for prevention of fouling in a basic solution by addition of specific nitrogen compounds. US Patent 4673489
Roling PV (1993) Method for inhibiting fouling in caustic scrubber systems. US Patent 5194143
Saeed AM, Mojtahedi MM, Pasha GF, Ghasem F, Akbarzadeh E, Shockravi A, Mesbah AW, Massa W (2011) Switching the reactivity of dihydrothiopyran-4-one with aldehydes by aqueous organocatalysis: Baylis–Hillman, aldol, or aldol condensation reactions. Org Lett 13:5282
Silvana P, Mauro DN, Domenico M, Daniele N, Ada N, Romualdo R (2011) Diastereo- and enantioselective direct aldol reactions in aqueous medium: a new highly efficient proline-sugar chimeric catalyst. Adv Syn Cat 353:1443
Subramaniyam M, Bankar P (2006) Method for prevention of fouling in basic solution by inhibiting polymerization and solubilizing deposits using amino acids. US Patent 697824
Tabler DC (1970) Removal of carbonyls from polymerizable monomers. US Patent 3535399
Tomoyuki M, Kouichi F, Yuuji K, Masaki T (1996) Aldehyde aldol condensation process. German Patent DE 19605078 A1 19960814
Wade LG (2005) Organic chemistry, 6th edn, Chap 18. Prentice Hall, Upper Saddle River, NJ, p 804
Zhu P, Chen X, Roberts CG (2006) Method of use for aldehyde removal. US Patent 7041220
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bhajiwala, H.M., Patil, H.R. & Gupta, V. Studies of amino acids for inhibition of aldol condensation and dissolution of polymeric product of aldehyde in alkaline media. Appl Petrochem Res 3, 17–23 (2013). https://doi.org/10.1007/s13203-013-0025-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-013-0025-y