Abstract
In the current study, the effect of CuO nanoparticles (CuO-NPs) at the presence of dodecyl-3-methylimidazolium chloride ([C12mim][Cl]) is investigated on the interfacial tension (IFT) reduction, wettability alteration, and even tertiary oil recovery. Since the prepared solutions with CuO-NPs are completely dark and it is impossible to measure the IFT of these solutions in the presence of crude oil using the pendant drop method (since one of the phases must be transparent for IFT measurement using the pendant drop method), n-heptane (representative of saturates) and toluene (representative of aromatics) are used only for IFT measurement of solutions prepared by CuO-NPs, while rest of the experiments are performed using crude oil. The obtained results reveal that CuO-NPs are not stable in the aqueous solution in the absence of surfactant which means fast precipitation of CuO-NPs and a high risk of pore plugging. In this way, the stability of CuO-NPs is investigated at the presence of dodecyl-3-methyl imidazolium chloride ([C12mim][Cl]) as an effective surfactant for stabilizing the CuO-NPs in the aqueous solution (more than 1 month without precipitation using 1000 ppm of IL). Further measurements reveal that although the presence of IL in the aqueous solution can reduce the IFT of oil/aqueous solution system, especially for the aqueous solutions prepared by formation brine (0.65 mN.m−1), the presence of CuO-NPs has no considerable effect on the IFT. On the other hand, not only the contact angle (CA) measurements reveal the considerable effect of IL on the wettability alteration toward water-wet condition (68.3° for IL concentration of 1000 ppm) but also the addition of CuO-NPs can significantly boost the wettability alteration toward strongly water-wet condition (23.4° for the concentration of 1000 ppm of CuO-NPs). Finally, several core flooding experiments are performed using different combinations of chemicals to find the effect of these chemicals on the tertiary oil recovery factor. The results reveal that the presence of CuO-NPs can enhance the oil recovery of injected chemical slug (aqueous solution prepared by dissolution of IL with an oil recovery factor of 10.1% based on Original oil in place (OOIP)) to 13.8, %, 16.9%, and 21.2% based on OOIP if 500, 1000, 2000 ppm of CuO-NPs existed in the solution concomitant with 1000 ppm of [C12mim][Cl].
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unfortunately, the majority of the running oil fields around the world are reaching to decline production phase regardless of efforts oil companies performed even using Enhanced oil recovery (EOR) methods such as low-salinity, chemical or water injection (Cheraghian, 2015). In detail, although several methods were utilized through the oil reservoirs, more than 50% of the original oil in palace (OOIP) remained trapped and unrecovered in the reservoirs (Abhishek et al., 2015). In this way, during the past decades, a large number of investigations were carried out attempting to recover more oil from the abandoned or depleted reservoirs using new techniques such as nanotechnology individually or concomitant with other potential EOR methods (Cheraghian and Hendraningrat, 2016). Nanotechnology is one of the latest innovative and worldwide approaches that utilizes NPs in the range of 1–100 nm for different purposes, especially improving the fluids rheological properties if they added to the base fluid (Bera and Belhaj, 2016; Khalil et al., 2017; Negin et al., 2016). Respecting the application of NPs in EOR industries, it is proven that it is possible to manipulate the IFT values (Ju et al., 2006; Torsater et al., 2012; Zaid et al., 2013), wettability of rock surface (Al-Anssari et al., 2016; Saien and Gorji, 2017), modify the viscosity of the crude oil and even leading to oil swelling (Ehtesabi et al., 2015; El-Diasty and Ragab, 2013; Kazemzadeh et al., 2015; Mohammadi et al., 2017; Taborda et al., 2016, 2017; Wei et al., 2007), nanoemulsion creation (Bobbo et al., 2012), pore channel plugging (Anganaei et al., 2014; Hashemi et al., 2013), and disjoining pressure (Aveyard et al., 2003; McElfresh et al., 2012). Using NPs at different conditions can even increase the thermal conductivity of the rocks using metal-based NPs which is highly desired for thermal-based EOR processes. The worth mentioning point is that in some cases it is reported that application of NPs in the system can activate multiple mechanisms such as wettability alteration and disjoining pressure consequently leading to higher sweep efficiency (McElfresh et al., 2012; Zamani et al., 2012). Besides the aforementioned advantages of NPs for oil recovery, the transportation efficiency of NPs through the porous media was also studied since not only NPs have the ability to move through the pore networks with the assist of their small sizes, but also they remained dispersed to some extent in the solutions in the shadow of their high active surface (Rodriguez Pin et al., 2009). Regarding the possible advantages and potentials of NPs, NPs-based methods are proposed as promising methods in EOR industries changing the wettability and reducing the IFT for a better mobility ratio (de Castro Dantas et al., 2017; Moradi et al., 2015; Suleimanov et al., 2011).
For example, Onyekonwu and Ogolo (Onyekonwu and Ogolo, 2010) studied the possible potential of some polysilicon NPs (PSNP) including lipophobic and hydrophilic (LHPN), hydrophilic and lipophobic (LHPN), and neutrally wet PSNP (NWPN) for EOR purposes with the main focus on the wettability alteration capability of these NPs. Also, Hendraningrat et al. (Cheraghian, 2016; Hendraningrat et al., 2013a, 2013b, 2013c; Torsater et al., 2012) claimed that using silica-based NPs introduce a considerable effect on the oil recovery by changing the rock surface wettability. Moreover, Shahrabadi et al. (Shahrabadi et al., 2012) performed a systematic investigation on the influence of hydrophobic and lipophilic polysilicon (HLP) NPs for higher oil production by changing the IFT and wettability. Their results revealed that using these NPs changed the CA from 123.34° to 95.44° and IFT values from 25.6 to 1.75 mN/m with an optimum concentration of 4 gr/ lit for HLP-NP (Shahrabadi et al., 2012). Furthermore, Mohammadi et al. (Seid Mohammadi et al., 2014) selected the Al2O3-NP as an EOR agent for the sandstone reservoir. Years after, Tarek (Tarek, 2015) proposed a new approach regarding the application of NPs which was a hybrid of several NPs instead of using only one NPs. In detail, Tarek reported that using a mixed solution of different NPs such as aluminum oxide (Al2O3), iron oxide (Fe2O3) and silicon oxide (SiO2) is more effective than the solution comprised of only one type of NPs.
The worth mentioning point is that before 2000, the main focus of the researchers was thermal advantages of NPs, while after 2000 the situation was different and the researchers were focused on the chemical advantages of NPs (Cheraghian and Hendraningrat, 2016; Sheng, 2010). In this way, the current investigation is focused on the application of metal-based NPs of CuO for chemical purposes such as IFT reduction and wettability alteration. In this way, not only CuO-NPs are used for EOR purposes in the current investigation, but also since there is no report regarding the effect of ionic liquid (IL)-based surfactants such as dodecyl-3-methyl imidazolium chloride ([C12mim][Cl]) on the stability of CuO-NPs during the IFT measurements and wettability alteration determination followed by core flooding experiments. The main idea of using [C12mim][Cl] as an effective surfactant for EOR purposes comes from different advantages of this type of chemicals including their potential for IFT reduction even if they dissolve in high salinity water, their effects on the NPs stability, and their possible potential for wettability alteration.
In general, ILs are a new substitution of conventional chemicals since they can provide unique characteristics such as lower melting and glass transition temperature (Dharaskar Swapnil, 2012; Domańska, 2005), being cost-effective and commercially available (Chen et al., 2014), being non-flammable, and have a wide range of solubility and miscibility (Lee and Kim, 2013; Martins et al., 2014; Peng et al., 2011), etc., especially for the researchers who are seeking for green solvents. Since these chemicals are comprised of two different sections (cationic and anionic groups), it is possible to tailor any type of IL with a specific characteristic that makes them a good candidate for different industries. In detail, ILs are molecules that can be tuned for any specific application such as EOR by changing the numbers of anions and cations combination (José-Alberto and Jorge, 2011; Khupse and Kumar, 2010).
For example, Smit et al. (Smit et al., 1991) and Hezave et al. (Hezave et al., 2013b) reported similar outcomes regarding the surface activity of ILs, especially if they are utilized in aqueous solution with high salinity. In detail, they have claimed that as the cationic IL are dissolved in saline water, the positive charges of cationic ILs can be neutralized by negatively charged ions resulting in the accumulation of ILs molecules at the oil–water interface leading to low IFT values. But the point that must be considered is that the IFT reduction using ILs is not low enough to consider the IFT reductions a dominant mechanism for oil recovery since they can only reduce the IFT up to 100–10−1mN/m (in special cases 10–2 mNm−1) (Hezave et al., 2013a). So it seems that individual application of ILs for IFT reduction is not satisfactory for oil recovery purposes (Rodríguez-Palmeiro et al., 2015).
In this way, the current investigation is aimed to use the [C12mim][Cl] as IL-based surfactants concomitant with CuO-NPs to modify the wettability of the rock surface and also reduce the IFT value to some extent for higher oil recovery. In this way, the pendant drop method was used to measure the IFT and measure the contact angle values at different concentrations of IL and CuO-NPs to find the optimum formulation. Finally, the optimum formulation was used to perform several core flooding experiments to find the potential of chemical formulation of tertiary oil recovery.
Materials and methods
Formation brine, crude oil and rock
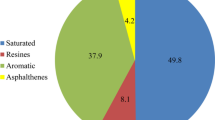
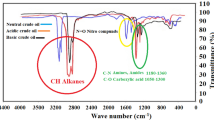
Bangestan heavy crude oil with APIo of 19.8 and formation brine (Na+ & K+ (76,833 ppm), Ca2+ (13,421 ppm), Mg2+ (1508 ppm), Fe2+ (69 ppm), Cl− (141,582 ppm), HCO3− (1620 ppm) and SO42− (1433 ppm)) were kindly provided by National Iranian south oil company (NISOC) and utilized. The used cores, the majority of which were dolomite, were prepared from outcrop rock having the same formation characteristics as the Bangestan oil field has (majority of its content is dolomite) (see Table 1).
Chemicals
The required CuO-NPs, n-heptane, and toluene (Sigma-Aldrich, USA, minimum purity of 99%) were used as the sample NPs and synthetic crude oil for the IFT and, CA measurements.
IFT and CA measurement
In this study, pendant drop equipment (Fanavari Atiyeh Pouyandegan Exir Co., Arak. Iran) (see Fig. 1)) was used to measure the IFT and CA values of different solutions.
The pendant drop method is a proper, simple and accurate method for IFT and CA measurements (Yang et al., 2014). The system is mainly comprised of three sections including image capturing and processing unit, automatic injection system, and visual measuring chamber providing the condition for a drop to be contacted with the bulk phase. After injecting the drop at the tip of the nozzle, it is possible to use the drop shape analysis approach (Eq. 1) to find the large and small dimeters and then calculate the IFT value using calculated shape factor (Stauffer, 1965).
where Δρ, g, and H are the difference between the bulk and drop phases, acceleration of gravity, and the shape-dependent parameter. The H value is depending on the shape factor value, i.e., S = d/D, where D is the equatorial diameter and d is the diameter at the distance D from the top of the drop. Also, the software is developed in a way that it is possible to calculate the contact angle of the drop at the captive or non-captive condition by injecting the oil drop on the top or beneath the surface of thin section.
The worth mentioning point is that the IFT between distilled water (DW) and n-heptane and distilled water and n-hexane was measured using the pendant drop equipment to ensure the purity of the chemicals and accuracy of the measurements. The obtained results revealed that the measured IFT @ 25° for DW/n-heptane and DW/n-toluene were 50.78 ± 0.4 mN.m−1 and 35.4 ± 0.4 mN.m−1, respectively, which is in good agreement with those reported by Zeppieri et al. (Zeppieri et al., 2001) and Saien and Akbari (Saien and Akbari, 2006) for DW/n-heptane = 51.24 mN.m−1 and DW/toluene = 35.8 mN.m−1.
Core flooding experiment
The used core flooding assembly in the current work was provided by Fanavari Atiyeh Pouyandegan Exir Co., Arak, Iran, rated for maximum pressure and temperature of 400 bar and 120 °C, respectively. The system comprising a pulseless injection pump enables the operator adjusting the injection flow rate at the desired value and pressure. The used injection flow rate was 0.3 cc/min for all of the flooding stages (oil flooding, formation brine flooding, chemical slug flooding, etc.) to mimic the laminar condition of flow movement in the porous media which is about 1 ft/day. The system comprises accumulators which can be used for injection of different fluids into the core in different stages, a hassler-type core holder to apply confining pressure well above the injection pressure, pressure transmitters to record the injection pressure, and a temperature controlling system to provide the required temperature for the experiments if needed (Fig. 2).
In detail, after placing the core inside the core holder and applying the desired confining pressure, the formation brine injection started for more than 5 pore volumes (PVs). After that, the crude oil flooding started for several PVs to the point no formation brine was produced (reaching the irreducible water saturation). At this point, the core is ready to be utilized for secondary oil recovery and tertiary oil recovery stages. During the secondary core flooding stage, the formation brine was injected for several pore volumes with an injection flow rate of 0.3 cc/min to the point in which the water cut reached 95%. After that, the chemical slug with the size of 0.3 PV was firstly injected with an injection flow rate of 0.3 cc/min and then chased with formation brine to the point in which no crude oil was produced. At this point, the production data can be used to calculate the oil recovery for secondary and tertiary stages.
Results and discussion
Effect of [C 12 mim][Cl] on the IFT
In the first stage, the effect of [C12mim][Cl] concentration in the range of 0–4000 ppm was investigated using the pendant drop IFT measurement technique (see Fig. 3). The measured IFT values revealed that as the IL concentration was increased from 0 to 250 ppm, a sharp reduction in IFT from 27.3 mN.m−1 to 2.1 mN.m−1 was observed, while further increase was observed in the concentration from 250 to 4000 ppm leading to a moderate reduction in IFT from 2.1 to 0.63 mN.m−1 (see Fig. 3). According to this observed trend, the concentration of 250 ppm can be considered as critical micelle concentration (CMC) for IL + aqueous solution/crude oil.
In detail, as the surfactant concentration increases, the surfactant molecules can be adsorbed at the surface till it is fully overlaid, leading to the minimum surface tension value. At this point, further dissolution of surfactant in the aqueous solution leads to micelles formation in the volume phase above the transition concentration generally known as CMC. Knowing the CMC values is important since knowledge of this parameter indicates the point where the higher concentration of surfactant has no meaningful effect on the IFT which directly can be utilized as the limiting concentration for meaningful use. According to these facts, it seems that the proper concentration to reach the minimum IFT value is 250 ppm while considering the real situation, especially in the core and porous media (adsorption of surfactant on the rock surface) leading to selection of higher concentration as an optimum value which requires more detailed analysis. By the way, the optimum concentration of examined IL was considered 250 ppm at this point although further analysis presented in the ongoing sections may manipulate this optimum value (Fig. 4).
Stability of the CuO-NPs
One of the most important parameters regarding the application of NPs in the EOR processes is their stability in the aqueous solution. In detail, any precipitation of NPs regardless of their type (metal-based or non-metal-based) may bring a catastrophic impact on the reservoir by plugging the pore networks which makes the trapped oil inaccessible forever. In this way, it is highly required to examine the stability of NPs dispersed in the aqueous solution. The analysis revealed that dispersing 100–2000 ppm of CuO-NPs without any surfactant led to rapid precipitation of CuO-NPs in the solution. Respecting this point, the effect of IL (100–4000 ppm) on the stability of CuO-NPs was investigated. According to the findings, it seems that increasing the IL concentration from 100 to 1000 ppm leads to a significant stability for more than one month, while further increase in the IL concentration to 4000 ppm leads to CuO-NPs precipitation. In other words, it can be concluded that there is an optimum concentration for IL which can provide ultimate stability for dispersed CuO-NPs in the aqueous solution. In this way, for the rest of the experiments, the concentration of IL was kept constant at 1000 ppm for rest of the experiments (Fig. 5).
Effect of CuO-NPs stabilized by [C 12 mim][Cl] on the IFT
In the third stage of this investigation, the effect of CuO-NPs was investigated on the possible IFT reduction. In this way, CuO-NPs solutions stabilized with 1000 ppm of IL were prepared with concentrations of 50, 100, 500, 1000, and 2000 ppm to find the effect of CuO-NPs on the IFT of aqueous solution/crude oil. Since the pendant drop method can calculate the IFT value if at least one of the phases is transparent and in the present case, both drop phase (crude oil) and bulk phase (CuO-NPs stabilized by IL) are dark, it is impossible to measure the IFT between these two systems using pendant drop method. For this purpose, instead of using crude oil utilized in the previous sections, two pure chemicals namely n-heptane and toluene were used as the synthetic oil for IFT measurements. Besides, in the conventional IFT measurements, the crude oil is considered as a drop, and the aqueous phase is considered as the bulk phase. But, in this section, the CuO-NPs solution stabilized with IL was considered as the drop and the aforementioned hydrocarbons were used as the bulk phase. In this way, several IFT measurements were performed using n-heptane, toluene, and a mixture of n-heptane + toluene (50/50 v/v), while the concentration of CuO-NPs was ranged between 50 and 2000 ppm. The measured IFT values for different solutions revealed that the dissolution of CuO-NPs led to a slight reduction in the IFT of n-heptane/aqueous solution (from 5.1 to minimum value of 4.8 mN.m−1 for 2000 ppm of CuO-NPs), toluene /aqueous solution (from 2.7 to 2.3 mN.m−1), and n-heptane + toluene/aqueous solution (from 3.3 mN.m−1 to 2.8 mN.m−1). According to these findings, it can be concluded that the effect of CuO-NPs on the IFT is negligible.
Effect of CuO-NPs on the CA
In the current section, the effect of CuO-NPs in the range of 500–2000 ppm was investigated on the wettability alteration. In normal cases, the effect of any additive can be directly examined on the wettability alteration. But in the present case, since the CuO-NPs were unstable and precipitate in the aqueous solution, the solution must be modified by 1000 ppm of IL to avoid any precipitation of CuO-NPs. In this way, in the first place, the wettability of rock surfaces after aging was measured using CA measurement. After that, the wettability alteration of rock surface was examined using an aqueous solution containing 1000 ppm IL to find the individual effect of IL on the wettability alteration (1000 ppm IL used as the optimum IL concentration to stabilize the CuO-NPs). So this is the difference between measured CA of an aqueous solution including IL and aqueous solution including CuO-NPs + IL indicates the sole effect of CuO-NPs on the wettability alteration.
In the first step of measurements, the CA values of the aged thin sections were measured which were in the range of 125°–133°. After that, the CA for one of the aged thin sections was measured at the presence of an aqueous solution comprised of 1000 ppm IL leading to CA of about 68.8° which is an obvious wettability alteration from oil-wet.to water-wet condition. In the third step of this section, the aged thin sections were contacted with the different CuO-NPs + IL solutions (CuO-NPs concentration was 500, 1000, and 2000 ppm) for a period of 30 days and then the CA was measured for these thin sections. The worth mentioning point is that the required bulk phase for CA measurement was an aqueous solution with 1000 ppm of IL since the presence of any CuO-NPs in the solution turn the solution into a dark solution makes it impossible to measure the CA. The obtained results revealed that increasing the CuO-NPs concentration from 500 to 1000 ppm can reduce the CA from 48.1° to 23.4°, while further increase in the CuO-NPs concentration to value of 2000 ppm leads to no significant impact on the wettability alteration. Moreover, according to these findings, it is obvious that the presence of CuO-NPs has a considerable effect on the wettability alteration. The most possible mechanism for the wettability alteration using NPs especially SiO2 and TiO2 (El-Diasty and Ragab, 2013) can be correlated with the disjoining pressure and wedge film theory (see Fig. 6). In detail, disjoining pressure is a pressure which can remove fluids attached to the rock surface due to adhesion force of fluids/solid surface (Jiang et al., 2017). The investigations, regardless of theoretical or experimental type, demonstrated that the nanofluids directly affect oil adsorption on a rock surface toward reducing pattern by entering a structural disjoining force (film) between the oil and the rock surface and then creating a wedge film structure on the rock surface which means a smaller NPs leading to stronger repulsion forces (Kopanichuk et al., 2017; Lim and Wasan, 2017). In detail, the entropy of nanofluids increases since the NPs have a considerable freedom in the nanofluids providing a good capability for NPs to rearrange in the nanofluid.
Nanoparticle structuring in the wedge film resulting in structural disjoining pressure gradient at the wedge vertex (Wasan et al., 2011)
The worth mentioning point is that since the available surface of thin section compared with the aqueous solution (CuO-NPs + IL) which surrounded it during the aging process is limited, it seems that increasing the concentration from 1000 to 3000 ppm has no meaningful effect on the wettability alteration (measured CA). The reason behind this trend is that all the accessible sites of thin section were saturated by the CuO-NPs if the concentration reaches 1000 ppm. In this way and to check this hypothesis, two flooding experiments were performed which included only injection of several pore volumes of two different aqueous solutions (1000 ppm CuO-NPs + 1000 ppm IL and 4000 ppm CuO-NPs + 1000 ppm IL) into two cores with similar length (6 cm) and close porosity (15.3% and 15.1%) (see Fig. 7). As it is obvious in Fig. 6, injection of aqueous solution with CuO-NPs concentration of 1000 ppm into the core leading to a completely transparent and free of CuO-NPs solution which means the core acted like a filter and all the CuO-NPs were adsorbed in the core pores and surface, while injection of 3000 ppm of CuO-NPs solution leads to an obvious dark solution which means the core was completely saturated and the excess CuO-NPs left the core. This finding is important since one can conclude that CuO-NPs may act as a sacrifice for IL consequently reducing the IL adsorption. At this point, the IFT reduction and wettability alteration mechanisms can reach the ultimate efficiency (adsorption of CuO-NPs leading wettability alteration and preventing IL adsorption on the rock surface which means better IFT reduction capability) leading to high tertiary oil recovery.
Core flooding experiments
In the last section of this investigation, four different core flooding experiments were performed to find the effect of proposed chemical formulations (see Table 2 and Fig. 8). In the first step of core flooding experiments, an aqueous solution with only 1000 ppm of IL was injected as the chemical formulation to find the individual effect of IL on the tertiary oil recovery. This experiment is required since the CuO-NPs solution injection must be performed by 1000 ppm IL to avoid any precipitation. In this way, it is required to know the individual effect of an aqueous solution of 1000 ppm of IL on the oil recovery and the effect of CuO-NPs + 1000 ppm of IL to quantify the individual effect of CuO-NPs on the tertiary oil recovery. In other words, the presence of IL in the aqueous solution can enhance the oil recovery due to IFT reduction and wettability alteration, while the CuO-NPs can increase the oil recovery only by changing the wettability alteration. The results revealed that injection of aqueous solution (Run#1) with IL concentration of 1000 ppm leads to tertiary oil recovery of about 10.1% based on original oil in place (OOIP). This obtained oil recovery can be correlated with the effect of IL on both wettability alteration and IFT reduction. In the next step, the CuO-NPs solutions with concentrations of 500 ppm to 2000 ppm were examined, while the concentration of IL was kept constant at 1000 ppm. The results revealed that the addition of CuO-NPs has a direct increasing effect on the tertiary oil recovery from 13.8 to 21.2% based on OOIP which can be considered as the effect of CuO-NPs on the wettability alteration. The other possible effect of CuO-NPs besides its effect on the wettability alteration was its adsorption on the rock surface consequently preventing the adsorption of IL on the rock surface leading to better efficiency of the IL existing in the solution.
In other words, one can conclude that the difference between tertiary oil recovery of Run#1 and Runs#2, 3, and 4 is not individually related to the effect of CuO-NPs on the wettability alteration and maybe it is related to the better efficiency of existed IL in the solution. Unfortunately, since it is impossible to inject CuO-NPs at different concentrations in the absence of IL, it is uncertain to conclude the differences between the aforementioned runs are purely related to the effect of used CuO-NPs or not. But, the point one can conclude in certain is that using this type of formulation (IL + CuO-NPs) has a profound effect on the tertiary oil recovery which can be considered as an innovative and hybrid EOR method for future applications.
Conclusions
In the current investigation, a nanotechnology-based method for EOR purposes is examined using different analyses. In this way, CuO-NPs were selected as the sample NPs and then stabilized by [C12mim][Cl] for safe injection of CuO-NPs into the core with minimum risk of precipitation. The performed experiment revealed that:
-
Increasing the IL concentration from 0 to 4000 ppm leads to IFT reduction from 27.3 to 0.65 mN.m−1, although the sharp reduction in IFT was observed for IL concentration of 250 ppm which can be considered as the CMC value of this system.
-
CuO-NPs are not stable in the aqueous solution and experienced precipitation in the solution, while the addition of 1000 ppm IL significantly enhances the stability of CuO-NPs for more than 1 month which can reduce the risk of CuO-NPs precipitation and pre plugging.
-
In contrast to the IL, CuO-NPs have no significant and considerable effect on the IFT reduction which means that the presence of CuO-NPs may lead to higher oil recovery only by changing the wettability of the rock surface.
-
The measured CAs revealed that although the considerable effect of IL on the wettability alteration toward water-wet condition (68.3° for IL concentration of 1000 ppm) was observed, the addition of CuO-NPs significantly boosted the wettability alteration toward strongly water-wet condition (23.4° for the concentration of 1000 ppm of CuO-NPs).
-
The results revealed that the presence of CuO-NPs can enhance the oil recovery of injected chemical slug (aqueous solution prepared by dissolution of IL with an oil recovery factor of 10.1% based on original oi in place (OOIP)) to 13.8, %, 16.9%, and 21.2% based on OOIP if 500, 1000, 2000 ppm of CuO-NPs existed in the solution concomitant with 1000 ppm of [C12mim][Cl].
References
Abhishek R, Kumar GS, Sapru R (2015) Wettability alteration in carbonate reservoirs using nanofluids. Pet Sci Technol 33(7):794–801
Al-Anssari S, Barifcani A, Wang S, Maxim L, Iglauer S (2016) Wettability alteration of oil-wet carbonate by silica nanofluid. J Colloid Interface Sci 461:435–442
Anganaei H, Pourabdollah K, Rostami A (2014) Experimental improvement of nano-enhanced oil recovery using nano-emulsions. Arab J Sci Eng 39(8):6453–6461
Aveyard R, Binks BP, Clint JH (2003) Emulsions stabilised solely by colloidal particles. Adv Coll Interface Sci 100:503–546
Bera A, Belhaj H (2016) Application of nanotechnology by means of nanoparticles and nanodispersions in oil recovery-A comprehensive review. J Nat Gas Sci Eng 34:1284–1309
Bobbo S et al (2012) Viscosity of water based SWCNH and TiO2 nanofluids. Exp Thermal Fluid Sci 36:65–71
Chen L et al (2014) Inexpensive ionic liquids:[HSO 4]−-based solvent production at bulk scale. Green Chem 16(6):3098–3106
Cheraghian G (2016) Effects of titanium dioxide nanoparticles on the efficiency of surfactant flooding of heavy oil in a glass micromodel. Pet Sci Technol 34(3):260–267
Cheraghian G, Hendraningrat L (2016) A review on applications of nanotechnology in the enhanced oil recovery part B: effects of nanoparticles on flooding. Int Nano Lett 6(1):1–10
Cheraghian, G., 2015. Effects of nanoparticles on wettability: A review on applications of nanotechnology in the enhanced Oil recovery.
de Castro Dantas TN, de Souza TTC, Neto AAD, de Alencar Moura MCP, de Barros Neto EL (2017) Experimental study of nanofluids applied in EOR processes. J Surfactants Deterg 20(5):1095–1104
Dharaskar Swapnil A (2012) Ionic liquids (a review): the green solvents for petroleum and hydrocarbon industries. Res J Chem Sci ISSN 2231:606X
Domańska U (2005) Solubilities and thermophysical properties of ionic liquids. Pure Appl Chem 77(3):543–557
Ehtesabi H, Ahadian MM, Taghikhani V (2015) Enhanced heavy oil recovery using TiO2 nanoparticles: investigation of deposition during transport in core plug. Energy Fuels 29(1):1–8
El-Diasty, A.I. and Ragab, A.M., 2013. Applications of nanotechnology in the oil & gas industry: Latest trends worldwide & future challenges in Egypt, North Africa Technical Conference and Exhibition. OnePetro.
Hashemi R, Nassar NN, Pereira Almao P (2013) Enhanced heavy oil recovery by in situ prepared ultradispersed multimetallic nanoparticles: A study of hot fluid flooding for Athabasca bitumen recovery. Energy Fuels 27(4):2194–2201
Hendraningrat, L., Li, S. and Torsæter, O., 2013a. Effect of some parameters influencing enhanced oil recovery process using silica nanoparticles: an experimental investigation, SPE Reservoir Characterization and Simulation Conference and Exhibition. OnePetro.
Hendraningrat, L., Li, S. and Torsaeter, O., 2013b. A coreflood investigation of nanofluid enhanced oil recovery in low-medium permeability Berea sandstone, SPE International Symposium on Oilfield Chemistry. OnePetro.
Hendraningrat, L., Li, S. and Torsaeter, O., 2013c. Enhancing oil recovery of low-permeability Berea sandstone through optimized nanofluids concentration, SPE enhanced oil recovery conference. OnePetro.
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013a) Dynamic interfacial tension behavior between heavy crude oil and ionic liquid solution (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl]+ distilled or saline water/heavy crude oil)) as a new surfactant. J Mol Liq 187:83–89
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013b) Investigating the effect of ionic liquid (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl])) on the water/oil interfacial tension as a novel surfactant. Colloids Surf, A 421:63–71
Jiang, R., Li, K. and Horne, R., 2017. A mechanism study of wettability and interfacial tension for EOR using silica nanoparticles, SPE Annual Technical Conference and Exhibition. OnePetro.
José-Alberto, M.-H. and Jorge, A., 2011. Current knowledge and potential applications of ionic liquids in the petroleum industry. Ionic liquids: applications and perspectives.
Ju B, Fan T, Ma M (2006) Enhanced oil recovery by flooding with hydrophilic nanoparticles. China Particuology 4(1):41–46
Kazemzadeh Y, Eshraghi SE, Sourani S, Reyhani M (2015) An interface-analyzing technique to evaluate the heavy oil swelling in presence of nickel oxide nanoparticles. J Mol Liq 211:553–559
Khalil M, Jan BM, Tong CW, Berawi MA (2017) Advanced nanomaterials in oil and gas industry: design, application and challenges. Appl Energy 191:287–310
Khupse, N.D. and Kumar, A., 2010. Ionic liquids: New materials with wide applications.
Kopanichuk IV, Vanin AA, Brodskaya EN (2017) Disjoining pressure and structure of a fluid confined between nanoscale surfaces. Colloids Surf, A 527:42–48
Lee Y-S, Kim D-W (2013) Cycling performance of lithium polymer cells assembled by in situ polymerization of a non-flammable ionic liquid monomer. Electrochim Acta 106:460–464
Lim S, Wasan D (2017) Structural disjoining pressure induced solid particle removal from solid substrates using nanofluids. J Colloid Interface Sci 500:96–104
Martins MA et al (2014) Impact of the cation symmetry on the mutual solubilities between water and imidazolium-based ionic liquids. Fluid Phase Equilib 375:161–167
McElfresh, P., Olguin, C. and Ector, D., 2012. The application of nanoparticle dispersions to remove paraffin and polymer filter cake damage, SPE International Symposium and Exhibition on Formation Damage Control. OnePetro.
Mohammadi M, Dadvar M, Dabir B (2017) TiO2/SiO2 nanofluids as novel inhibitors for the stability of asphaltene particles in crude oil: Mechanistic understanding, screening, modeling, and optimization. J Mol Liq 238:326–340
Moradi B, Pourafshary P, Jalali F, Mohammadi M, Emadi M (2015) Experimental study of water-based nanofluid alternating gas injection as a novel enhanced oil-recovery method in oil-wet carbonate reservoirs. J Nat Gas Sci Eng 27:64–73
Negin C, Ali S, Xie Q (2016) Application of nanotechnology for enhancing oil recovery–A review. Petroleum 2(4):324–333
Onyekonwu, M.O. and Ogolo, N.A., 2010. Investigating the use of nanoparticles in enhancing oil recovery, Nigeria Annual international conference and exhibition. OnePetro.
Peng X-M, Hu Y-F, Jin C-W (2011) Solubilities of imidazolium-based ionic liquids in aqueous salt solutions at 29815 K. J Chem Thermodyn 43(8):1174–1177
Rodriguez Pin E, Roberts M, Yu H, Huh C, Bryant SL (2009) Enhanced migration of surface-treated nanoparticles in sedimentary rocks. Society of Petroleum Engineers, SPE annual technical conference and exhibition
Rodríguez-Palmeiro I, Rodríguez-Escontrela I, Rodríguez O, Arce A, Soto A (2015) Characterization and interfacial properties of the surfactant ionic liquid 1-dodecyl-3-methyl imidazolium acetate for enhanced oil recovery. RSC Adv 5(47):37392–37398
Saien J, Akbari S (2006) Interfacial tension of toluene+ water+ sodium dodecyl sulfate from (20 to 50) C and pH between 4 and 9. J Chem Eng Data 51(5):1832–1835
Saien J, Gorji AM (2017) Simultaneous adsorption of CTAB surfactant and magnetite nanoparticles on the interfacial tension of n-hexane–water. J Mol Liq 242:1027–1034
Seid Mohammadi M, Moghadasi J, Naseri S (2014) An experimental investigation of wettability alteration in carbonate reservoir using γ-Al2O3 nanoparticles. Iranian J Oil Gas Sci Technol 3(2):18–26
Shahrabadi, A., Bagherzadeh, H., Roustaei, A. and Golghanddashti, H., 2012. Experimental investigation of HLP nanofluid potential to enhance oil recovery: A mechanistic approach, SPE International Oilfield Nanotechnology Conference and Exhibition. OnePetro.
Sheng, J.J., 2010. Modern chemical enhanced oil recovery: theory and practice. Gulf Professional Publishing.
Smit B et al (1991) Structure of a water/oil interface in the presence of micelles: a computer simulation study. J Phys Chem 95(16):6361–6368
Stauffer CE (1965) The measurement of surface tension by the pendant drop technique. J Phys Chem 69(6):1933–1938
Suleimanov BA, Ismailov F, Veliyev E (2011) Nanofluid for enhanced oil recovery. J Petrol Sci Eng 78(2):431–437
Taborda EA, Franco CA, Lopera SH, Alvarado V, Cortés FB (2016) Effect of nanoparticles/nanofluids on the rheology of heavy crude oil and its mobility on porous media at reservoir conditions. Fuel 184:222–232
Taborda EA, Franco CA, Ruiz MA, Alvarado V, Cortes FB (2017) Experimental and theoretical study of viscosity reduction in heavy crude oils by addition of nanoparticles. Energy Fuels 31(2):1329–1338
Tarek, M., 2015. Investigating nano-fluid mixture effects to enhance oil recovery, SPE Annual Technical Conference and Exhibition. OnePetro.
Torsater O, Engeset B, Hendraningrat L, Suwarno S (2012) Improved oil recovery by nanofluids flooding: an experimental study. Society of Petroleum Engineers, SPE Kuwait international petroleum conference and exhibition
Wasan D, Nikolov A, Kondiparty K (2011) The wetting and spreading of nanofluids on solids: Role of the structural disjoining pressure. Curr Opin Colloid Interface Sci 16(4):344–349
Wei L, Zhu J-H, Qi J-H (2007) Application of nano-nickel catalyst in the viscosity reduction of Liaohe extra-heavy oil by aqua-thermolysis. J Fuel Chem Technol 35(2):176–180
Yang Z et al (2014) Interfacial tension of CO2 and organic liquid under high pressure and temperature. Chin J Chem Eng 22(11–12):1302–1306
Zaid HM, Yahya N, Latiff NRA (2013) The effect of nanoparticles crystallite size on the recovery efficiency in dielectric nanofluid flooding, Journal of Nano Research. Trans Tech Publ 21:103–108
Zamani A, Maini B, Pereira-Almao P (2012) Flow of nanodispersed catalyst particles through porous media: Effect of permeability and temperature. Canadian J Chem Eng 90(2):304–314
Zeppieri S, Rodríguez J, López de Ramos A (2001) Interfacial tension of alkane+ water systems. J Chem Eng Data 46(5):1086–1088
Acknowledgements
The current manuscript is only submitted to the journal of “Journal of Petroleum Exploration and Production Technology” and would not be submitted to the other journal when the decision regarding this submission completed. The current manuscript is original and performed by the authors of the manuscript in a clear way to enlighten concerns existed in the area of enhanced oil recovery based on chemical injection. Besides, it is not published elsewhere in any form or language (partially or in full) and is an expansion of our previous researches. It is not extracted from one study, and it is published from a series of studies performed regarding the EOR concerns. The obtained results and used procedure in the current manuscript are clearly stated and discussed make it easy for the readers to follow the current work. No data, text, or theories by others are presented as if they were the author’s own (‘plagiarism’). Proper acknowledgements are brought regarding the other works (this includes material that is closely copied (near verbatim), summarized and/or paraphrased), and quotation marks (to indicate words taken from another source) are used for verbatim copying of material and permissions secured for material that is copyrighted.
Funding
The authors declare that there are no funding resources for the current manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbood, N.K., obeidavi, A. & Hosseini, S. Investigation on the effect of CuO nanoparticles on the IFT and wettability alteration at the presence of [C12mim][Cl] during enhanced oil recovery processes. J Petrol Explor Prod Technol 12, 1855–1866 (2022). https://doi.org/10.1007/s13202-021-01441-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-021-01441-6