Abstract
Recently, smart water (SmW) and nanocomposite (NC) flooding have shown good potential for enhanced oil recovery (EOR) processes. Although SmW and NCs are found to influence the oil recovery, there are still some controversies regarding their performance on wettability alteration (WA). To address this important question, we synthesized new NC materials with high hydrophilic properties. In this research, in the first stage, a NC was synthesized. The obtained compound was known as (UiO-66-NH2/TiO2/ZnO) (UNTZ). For the identification of NC, scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET), Fourier transform infrared spectroscopy (FTIR), and X-ray Diffraction (XRD) techniques were used. Furthermore, zeta-potential analysis was done to investigate the stability of nanofluids (NFs). To investigate the effect of NFs on oil recovery, 8 concentrations (1600, 1400, 1200, 900, 700, 500, 300, and 100 ppm) of UNTZ nanofluids were prepared. In the current work, to investigate the effectiveness of the combination of SmW (sulfate (SO42−) and calcium (Ca2+)) + NCs, tests such as interfacial tension (IFT), contact angle, and coreflooding were used. The results of contact angle tests showed improved SmWs capabilities in the presence of NCs that a very effective reduction was accessible and highly hydrophilic wettability was obtained when using SmWs with stable NC as a minimum contact angle of 28° was achieved. The findings of the coreflood experiment indicated that at SmW(SmW2sulfate) + 100 ppm NC and SmW(SmW2calcium) + 100 ppm NC concentrations, the NF enhanced the oil recovery by 9.8 and 5.9%, respectively. This research offers new findings that can help oil recovery by understanding smart water technology with nanoparticle (NPs) in reservoirs (carbonate).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enhanced oil recovery (EOR) from carbonate reservoirs can be a great challenge in many cases. Generally, carbonate reservoirs are mostly oil-wet (OW) (Jafarbeigi et al. 2022a, 2023). After primary and secondary production (water injection), two-thirds of the oil typically remains in carbonate reservoirs. In general, chemical, thermal, and microbiological processes are only a few examples of the various EOR techniques. In the past, injection of some materials in oil reservoirs has always been known as a challenge because of the cost (Ghalamizade Elyaderani et al. 2019; Ali et al. 2020; Franco et al. 2021). On the other hand, the loss of injected materials during displacement in the reservoir has limited chemical floodings, such as the injection of alkaline or similar chemicals (Engeset 2012; Ghalamizade Elyaderani et al. 2019). Chemical enhanced oil recovery (CEOR) methods have been used by providing mechanisms such as reducing interfacial tension (IFT) and wettability alteration (WA) (Rostami et al. 2019; Farhadi et al. 2020; Lopez et al. 2020; Ahmadi and Mansouri 2021; Koleini et al. 2021; Ayirala et al. 2022a; Hassan et al. 2022; Nourinia et al. 2022). The use of nanotechnology has opened a significant field in the field of EOR (Jafarbeigi et al. 2022b; Mansouri et al. 2022; Soleimani et al. 2022; Ahmadi et al. 2022a, b). Most of the nanoparticles (NPs) considered for EOR applications are environmentally friendly materials compared to traditionally used chemicals (Jafarbeigi et al. 2022c). By changing the wettability from OW to WW, for example, nanofluids (NFs) improve the efficiency of oil reservoirs through various methods (Suleimanov et al. 2011; Gharibshahi et al. 2015). The injection of NPs into the reservoir converts the wettability of the rock surface from OW to WW, thereby facilitating the movement of oil within the porous media reservoir (Jafarbeigi et al. 2020a, 2021). Certain NPs have been employed to improve injected water into oil reservoirs to improve oil recovery. Numerous injectable waters, including smart/engineered water with unique salinities, can use these nano additives (Alghamdi et al. 2019; Hosseini et al. 2020; Ayirala et al. 2022b; Nowrouzi et al. 2022). Recently, many studies have investigated low-salinity water flooding to improve oil recovery since it can modify wettability and IFT (saw et al. 2022). Also, studies have shown that tuning of the ionic composition of seawater by keeping the ionic strength constant and successive dilution of seawater/formation brine are responsible for WA in carbonates during LWSF (Mahani et al. 2015; Saw and Mandal 2020; Namaee-Ghasemi et al. 2023). In addation, in an experimental effort, SiO2, MgO, Al2O3, ZrO2, CaCO3, TiO2, CeO2, and CNT on the wettability of CRs were explored by Nazari Moghaddam et al. (2015). They achieved acceptable results in their study. Rasooli-Manesh et al. (2017) studied the potential role of γAl2O3 NPs in increased oil recovery. They increased oil recovery by 4% by adding 0.3wt.% of Al2O3 NPs to the base fluid. In the past, the effects of Ziziphus spina-christi leaf extracts on interfacial tension reduction and EOR in carbonate reservoirs were investigated by Ahmadi and Shadizadeh (2013). The obtained data demonstrated that, due to a decrease in IFT, enhanced oil recovery with increasing surfactant content. Taborda et al. (2021) evaluated the impact of the silica NPs surface acidity for reducing the time-dependent thermal degradation of polymer solutions of partially hydrolyzed polyacrylamide for chemically EOR processes. With the use of NaOH(SiO2B) and HCl(SiO2A), they altered the surface of silica NPs. SiO2B NPs demonstrated even better interactions between the surface functional groups and the hydrolyzed polyacrylamide in the solution. With the addition of SiO2B and unmodified silicon dioxide NPs, viscosity was reduced by up to 22.9 and 49.6%, respectively. Additionally, compared to polymer without NPs, the aged polymer solution encourages a 30% greater oil recovery. In a laboratory study, Kumar and Mandal (2018a, b) came to this conclusion that SiO2 NPs can improve the stability and viscosity of nano-emulsions containing mineral oil/polymeric surfactant solution (poly(methyl ester sulfonate)). In another study, Kumar and Mandal (2018a, b) and Pal et al. (2019) get similar results about the impact of SiO2 NPs on the viscosity and stability of water/oil nano-emulsions. Kumar et al. (2016) investigated the effects of cationic surfactants from the trimethylammonium bromide on the IFT, and WA of CR were investigated. They indicated that hexadecyltrimethylammonium bromide and dodecyltrimethylammonium bromide surfactants yielded lower IFT and greater WA than tetrabutylammonium bromide and benzyltrimethylammonium bromide the higher the carbon atom, the higher the decrease in the critical micelle concentration of the surfactant. Khaksar Menshad et al. (2022) prepared a nanocomposite (NC) containing zinc and bentonite as natural clay in a simple, economical, and green way from the extract of the Cordyline fruticosa plant. They chose to assess the impact of prepared NCs dispersed in water at various salinity levels, such as saltwater and seawater. According to the results, the generated nanofluids are very stable, and as the concentration of NCs increases, the IFT and contact angle decrease. Their findings demonstrated that 62.14% of the original oil in place (OOIP) could be extracted by DIW-based nanofluid during secondary recovery. However, NCs increased oil recovery from 44 to 65.41%OOIP as a tertiary recovery technique. Also, Ali et al. (2021) prepared TiO2/SiO2/poly(acrylamide) NCs by using a green, simple and economical method from pomegranate seed extract. They prepared nano smart solutions from the dispersion of NCs in Smart water (SmW). The experimental results obtained from IFT and contact angle showed that the selected SmW solutions could reduce the IFT and provide an acceptable WA. In the end, the results indicated that the most oil recovery is obtained through this solution. In their study, the most effective method for enhancing oil recovery from 36.0 to 46.53% OOIP was shown by the Nano-OSS3-5000 smart-NF. Suleimanov et al. (2022) investigated low-salinity water flooding and NF flooding of oil reservoirs to enhance oil recovery and move trapped residual oil more effectively. They concluded that the developed NFs could increase oil recovery by 15–20%.
Generally, the purpose of this research was to investigate the use of a synthetic UiO-66-NH2/TiO2/ZnO NC in enhanced oil recovery procedures. By analyzing core during the injection of SmW + NC, we show how the presence of SmW + NC could increase the amount of oil recovery. In this experimental study, the combined method of NF and SmW was used to examine how water chemistry, namely ionic exchange (Sulfate and Calcium) of brine, affects oil recovery factors. In this investigation, various calcium and sulfate concentrations were employed (0 and 2 times). In order to determine how wettability altered, contact angle measurement was used on portions of the carbonate core plug. The oil recovery factor was finally studied by flooding through a CR. To the best of the authors' knowledge, there is no similar research in which all EOR-related parameters are examined simultaneously. Thus, as an innovation to alter the wettability of the CR, this new combination can be effective in the EOR. The more significant impact of these SmW + NC is due to creating a wedge-shaped layer by the disjoining pressure gradient between the rock surface and the oil.
Materials and methods
Materials
In this study, Table 1 lists the compounds that were employed. Every compound was analytical grade (assay: 99%). All of the trials were conducted using distilled water. The oil sample used was prepared from one of the oil reservoirs in western Iran. The crude oil used has ρ = 0.831 gr/cm3, μ = 15.41 cp, and API = 38.7. Two CR samples were used for experiments. Table 2 shows the properties of CRs. Also, SARA analysis data of the studied crude oil are reported in Fig. 1. A FESEM equipped with EDX analysis is applied to identify the carbonate minerals. Generally, according to Table 3, CR rock contains elements such as aluminum, iron, silicon, carbon, potassium, titanium, and oxygen.
Instruments
FTIR (460 Plus Jasco spectrophotometer, USA) and XRD (Philips PW 1800) techniques were utilized to ascertain the structural characteristics. Additionally, the morphological features were evaluated using a transmission electron microscopy and a scanning electron microscope (Leo 1455 VP). Materials' elemental composition was determined using energy-dispersive X-ray analysis. An OPTIZEN3220UV UV/visible spectrophotometer and a ζ-sizer nano (Malvern Instruments Ltd, Malvern, UK) were used to ensure the stability of the NFs. Also, the Quanta chrome Nova 2000 automated system was used to measure the Brunauer–Emmett–Teller (BET) surface areas, pore volumes, and average pore sizes of the used samples.
Preparation of the NCs
In this study, UiO-66-NH2/TiO2/ZnO NC was synthesized in several steps. In general, the preparation steps of NC in Fig. S1 are reporThe qualitative stability of NFs at difted step by step.
Fluids
In this section, some synthetic seawater was created to assess the effects of calcium and sulfate ions at various concentrations (0 and 2 times). Table 4 displays the molar compositions of several brines used in contact angle and coreflooding experiments. (SmWAcalcium where A shows A times the calcium concentration of seawater.) In this research, NF is only stable at a concentration of 100 ppm in designated synthetic seawater. In Sect. 3.2, the data indicate this issue. As a result, the brines now contain 100 ppm of NF. Thus, SmW and SmW with NF were prepared as the main test solutions. The ionic strength of brines was assumed to be equal to that of seawater in order to compare the impact of ions on the oil recovery factor. Additionally, NaCl was employed to modify the ionic strength.
Stability analysis
The stability of NF with SmW solutions has been studied using two quantitative and qualitative approaches. In the qualitative investigations, the prepared desired solution was kept out of light and heat for a few hours to several days before being placed in a tiny, transparent container and photographed at various intervals. In terms of stability, a solution was seen to be stable if there was little to no deposition and no noticeable color change. The UV–visible spectrophotometer was used to quantify the quantity of light absorption going via the solutions at various times. In other words, distilled water (DIW) has 0% light absorption at any wavelength. Thus, as more material is deposited, the amount of light absorption falls until it is similar to that of pure water.
Interfacial tension (IFT) and contact angle measurements
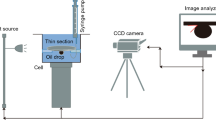
The pendant drop method was used to test the IFT of oil/water in the presence of UNTZ NCs and SmW. Also, contact angle measurement was used to assess the rock wettability. For this purpose, initially, coin-shaped slices with a thickness of 2 mm were cut from the CR. Then, they were washed with toluene (7 days), and DIW (4 days). Following a 2-week aging process in crude oil, the plates were finally immersed vertically for 24 h in the ready solutions (Jafarbeigi et al. 2020b). Then, into the cell holding the aqueous phase was gently injected a drop of oil to measure the contact angle. Then, a camera was connected to the CR surface to capture a photo of the oil droplet, and the image was then accurately measured to within one degree. Figure 2 depicts the schematic for this device.
Coreflooding experiments
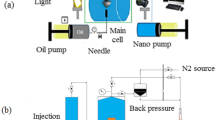
In this research, the flood system includes 3 pressure cylinders to transport the fluid. These fluids can be seawater or SmW, crude oil, and NF. In Fig. S2, the procedure of coreflooding is reported step by step. Also, Fig. 3 shows the components of this system.
Schematic of coreflooding apparatus (1—Injection pipe, 2—Pump fluid, 3—Pump, 4—Valve, 5—Seawater or SmW in vessel, 6—Nanofluid in vessel, 7—Crude oil in vessel, 8—Seawater pipeline, 9—Nanofluid pipeline, 10—Oil pipe, 11—By pass valve, 12—Inlet pipeline, 13—Hassler core cell inside oven, 14—Carbonate core plug, 15—Sleeve pressure, 16—Pressure transmitter, 17—Camera, 18—Oil–Water separator, 19—Water accumulator, 20—Connection cable to PC, 21—PC/Computer as controller and data acquisition)
Results and discussion
NC Characterization
In this study, all the samples were characterized using XRD, SEM observations, EDX qualitative analysis, FTIR spectroscopy, and TEM observation. Thus, at first, to confirm the structure of the synthesized NC and the identification of novel compounds on its structure, the XRD spectrum was taken. In this regard, UNTZ and UiO-66-NH2 NPs XRD patterns are shown in Fig. 4. As can be observed, the normal pattern recorded for UN and the simulated one (CCDC No. 889529) matched with all peaks emerging at 7.32°, 8.92°, 17.33°, 21.11°, 27.31°, and 40.06°. The ZnO peaks at 2θ = 32.95°, 35.22°, 37.61°, 48.8°, and 72.69° which correspond to the lattice planes of (100), (002), (101), (102), and (004), respectively. In this regard, Fig. 4 confirms the hexagonal wurtzite ZnO structure that was successfully synthesized. Moreover, titania particles were detected according to Mansouri et al. (2017) group (2θ = 58.9° and 77.6°). This shows the formation of ZnO and TiO2 nanoparticles over the synthesized UNTZ NCs. The sharp intense peaks of UNTZ NCs confirmed the high crystalline structure of the sample. Debye Scherrer equation showed that the average crystallite size of the synthesized UNTZ nanoparticles is 33.76 nm.
Also, the FTIR results for UNTZ samples are displayed in Fig. 5. The stretching vibration of Zn–O can be attributed to the absorption band at 436 cm−1 (Karkare 2014). The stretching vibrations of Ti–O are represented by the bands at 662 and 770 cm−1 (Soni et al. 2013). Additionally, the band that appears at 831 cm−1 is caused by the stretching vibration of Zn–O–Ti. Ti–O and Ti–O–C in signals can be attributed to the broad absorption band centered at1254 cm−1 (Moradi et al. 2012; Mofokeng et al. 2017). Moreover, carboxylic groups' asymmetric and symmetric stretching vibrations (C(=O)OH) are responsible for the signals at 1432 and 1572 cm−1. Also, the stretching vibrations of 'C=O' and 'C=C' of MOF are also indicated by the sharp bands at 1658 and 1380 cm−1 (Moradi et al. 2012). The presence of carboxylate groups in the MOFs' organic linkers may also cause the band at 1254 cm−1. A band at 3350 cm−1 corresponds to the stretching vibrations of the 'N–H' signal in terephthalic amino acid (or other compound containing –NH2). In contrast, the band at 3480 cm−1 is also seen due to the stretching vibrations of the 'OH' signal, which is related to water molecules (Li et al. 2018; Strauss et al. 2020). SEM morphological images of the synthesized UNTZ structure are shown in Fig. 6. Figure 6a and b shows scanning electron microscope and transmission electron microscopy pictures of UNTZ NC. According to the scanning electron microscope morphology, the average particle size of UNTZ NC was around 33.63 nm. According to Yang et al. (2019) presentation s of the scanning electron microscope morphology of UN, the particles are nanometer-sized, with a distribution between 40 and 90 nm (Yang et al. 2014). On the other hand, the transmission electron microscopy picture of various generated nanorods related to ZnO is shown in Fig. 6b. The chemical composition of UNTZ and UiO-66-NH2 was evaluated using the energy-dispersive X-ray analysis method, as shown in Fig. 7a, which verifies the existence of nitrogen, carbon, zirconium, and oxygen at corresponding molar percentages of 58.19, 5.28, 33.43, and 3.1. The principal diffraction peaks for zirconium, nitrogen, carbon, and oxygen are also visible in the energy-dispersive X-ray analysis image of UN, indicating that the synthesis of UN was successful. Ti and Zn are found in UNTZ NC with molar percentages of 13.61 and 18.58, respectively, as can be observed in Fig. 7b. Additionally, nitrojen, carbon, zirconium, and oxygen can be seen. The inclusion of TZ NPs into UN is suggested by the Energy Dispersive X-Ray Analysis picture of UNTZ, which displays a second diffraction peak connected to titanium and zirconium. The absence of any impurity peaks in the Energy Dispersive X-Ray analysis images reflects the cleanliness of the samples.
Nanofluid stability
The qualitative stability of NFs at different concentrations (1600, 1400, 1200, 900, 700, 500, 300, and 100 ppm) in conjunction with the brines was discussed in this part. Due to their higher concentrations of sulfate and calcium in manufactured brines, SmW2sulfate and SmW2calcium induced increased instability in solutions. The findings demonstrate that, with the exception of values of 100 and 300 ppm, the NFs were unstable at all concentrations and immediately formed white sediment. Generally, the NF at a concentration of 100 ppm in synthetic seawater started to precipitate a bit after 30 h. At a concentration of 100 ppm, it remained stable for 3 days without any sediment forming.
Additionally, the NFs stability (at 100 ppm) was examined over the course of a week. And in this regard, the results showed that NF is stable at this concentration. In general, the results showed that NP aggregation happens right away following the production of these solutions (various concentrations of NFs with SmW2calcium). On the other hand, Ca2+ ions are drawn to the negatively charged NPs and lower their negative surface charge as a result. Negatively charged divalent cations like calcium can effectively neutralize NPs. As a result, van der Waals (vdW) forces between particles become stronger than electrostatic forces that are repellent, which leads to "agglomeration and in-stability" of the solutions (Kobayashi et al. 2005). The diagram for this mechanism is shown in Fig. 8. In order to check the stability of nanoparticles in salt water, a dynamic light scattering measurement was performed. Also, to examine the changes in average particle size brought on by natural precipitation, the solutions (300-SmW0calcium, 300-SmW2calcium, 300-SmW0sulfate, 300-SmW2sulfate, 100-SmW0calcium, 100-SmW2calcium, 100 SmW0sulfate, 300 and 100 show the concentration of NF in ppm) were measured at 1, 3, and 7 days (30, 80, 240 h) apart without shaking. The diameter of the NPs typically decreased from the first to the last day of measurement in unstable solutions due to the deposition of bigger particles. On the seventh day, these solutions totally precipitated, and the concentration of particles in suspension was lowered to a level that was not detectable, making the average diameter of the particles in solutions containing 100 ppm of NF only measurably in the first 2 days. As a result, the mean particle diameter shrunk with time, and only smaller particles remained in the solution while bigger ones precipitated at the bottom of the container. Table 5 shows that solutions with 100 ppm of NF remained remarkably stable. In other words, the average size determined by DLS on days 1 and 7 was nearly identical. Figure 9 shows the 300-SmW2sulfate solution's particle size distribution at 1 h, 1 day, and 3 days. Additionally, the quantitative stability of the solutions demonstrates that at 300 ppm with the designated synthetic seawater, the number of NFs that absorbs light is significantly reduced. These solutions consequently destabilize quickly, supporting the qualitative findings. Figure 10a illustrates these findings. At a concentration of 100 ppm, the change in the amount of light that NFs absorb is not particularly noticeable, as shown in Fig. 10b, and the solution is stable. Therefore, 100 ppm of the NF was chosen to guarantee that the injected fluid is stable over the coreflooding period. The application of DLVO (Derjaguin and Landau, Verwey and Overbeek) theory justifies a solution's stability. When the electrolyte concentration rises, van der Waals gravity forces increase, and repulsion forces between the particles decrease. As a result, NPs start to absorb each other and finally start sediment. In the presence of high NF concentrations and brines, interactions between the particles and these forces intensify, making the solution more unstable.
Contact angle and IFT measurements
In this section, the wettability of CRs in the presence of synthetic seawater is reported. So, in this regard, these salts have been investigated with 100 ppm NF. The results of these measurements in salt water and salt water containing NF solution are shown in Fig. 11, respectively. These results were made after 80 h. As shown in Fig. 11a, the brine solution's capacity to modify wettability is increased by adding calcium ions (SmW0calcium, SmW2calcium). To put it another way, the sulfate ion as one of the Ca2+ ion pairs cannot alter the wettability of the medium on its own in the absence of a Ca2+ ion, and as a result, the improvement in the oil recovery factor is insignificant. As seen in Fig. 11b, adding NF to saltwater solutions causes the contact angle to drop significantly, indicating how powerfully the NF may alter the wettability and shift the surface toward wetness. According to RezaeiDoust et al. (2009) SO42− ions are hydrated in water due to their hydrogen bonds. At high temperatures, the reactivity of SO42− ions increases due to the breaking of hydrogen bonds on the rock surface. As a result, SO42− ions are absorbed more strongly on the CR surface. This approach reduces the repulsive forces between the positive charges on the rock surface and the cations (RezaeiDoust et al. 2009). As shown in Fig. 11, the contact angle decreases noticeably as sulfate ion concentration is increased. Additionally, Fig. 11b shows how adding NF to artificial seawater solutions (SmW0sulfate and SmW2sulfate) significantly reduces the contact angle and converts OW surfaces into highly WW surfaces. The NFs negative charge aids the sulfate ions ability to balance the surfaces positive charge. On the other hand, calcium ions move closer to the surface and can more easily separate oil droplets. The data obtained show that NF and brine with a doubled concentration of sulfate ions have the biggest effects on lowering the contact angle and up to 38° of surface wetness.
According to Fig. 12, the concentration of SmW2Sulfate is known as the effective concentration for wettability rock to water-wetting conditions with the most changes in the contact angle of the rock.
In this study, the effect of synthetic seawater and NC is well reported, so in this regard, the interfacial tension values are shown in Fig. 13. This diagram shows that the IFT is not affected by the ionic makeup of SmWs. Additionally, the IFT values are decreased in SmW + 100 ppm NC. Comparing the contact angle results with the IFT values shows that this NC has better potential in WA rock than IFT reduction (Fig. 14).
Coreflooding and ionic analysis
In this research, coreflooding tests were conducted in three steps using seawater, SmW, and NF (SmW + NC). To assess the impact of varied calcium ion concentrations, core samples 1 and 2 were utilized. The total recovery after injection of about 4 PVs of the seawater dispersions was 45 and 43%. Additional oil recovery that was observed from the injection of SmW(SmW2Sulfate) and SmW(SmW2calcium) were 10 and 12%, respectively. On the other hand, the total recovery after injection of the SmW(SmW2Sulfate) and SmW(SmW2calcium) were 55 and 53%. Thus, additional oil recovery that was observed from the injection of SmW(SmW2Sulfate) + 100 ppm NC and SmW(SmW2calcium) + 100 ppm NC were 6 and 3.5%, respectively. It shows the efficiency of the SmW(SmW2Sulfate) + 100 ppm NC in EOR. According to the results, displayed an excellent capability that made oil molecules displace from an OW surface. The calculated values of oil recovery ratio after SmW(SmW2Sulfate) and SmW(SmW2Sulfate) + 100 ppm NC flooding to overall oil recovery are reported in Table 6. Also, the calculated values of oil recovery ratio after SmW(SmW2calcium) and SmW(SmW2calcium) + 100 ppm NC flooding to overall oil recovery are reported in Table 7. It can be observed that 9.8 (SmW(SmW2Sulfate) + 100 ppm NC) and 5.9% (SmW(SmW2calcium) + 100 ppm NC) of the recovered oil in the core samples 1 and 2 is due to using SmW + NC. Every time NC was added to the solution (SmW), the oil recovery factor increase. Compared to other brines, SmW2sulfate has a higher increase in oil recovery. These findings are in line with the conclusions drawn from the contact angle section. As seen, adding NC makes the CR surface significantly more WW. In general, according to the findings, the reduction of sulfate ion concentration in the output indicated that these ions were absorbed to the surface of CR with a positive charge. Ca2+ ions tend to approach the CR surface due to the reduction of the net positive charge on the CR surface. This displacement approach in the porous media causes the Ca2+ ions to attach to the negatively charged carboxylic groups of the oil and separate them from the surface (Jafarbeigi et al. 2021). On the other hand, in this study, Ca2+ carbonate is dissolved in salt water to balance the Ca2+ concentration. And in this regard, it creates a mechanism in the porous media of the reservoir. This phenomenon is well shown in Fig. 15. When this mechanism takes place, more Ca2+ ions are released. Ca2+ ions tend to bind to carboxyl groups. Therefore, at the end of this mechanism, it can be seen that more oil can be produced from the reservoir.
Conclusions
This study aims to provide an understanding and assess the effect of a new synthetic nanocomposite (NC) with smart water (SmW). In this experimental study, an effective synthetic hydrophilic NC has been used to minimize the oil-wetting problem of the rock (carbonate). The obtained NC was known as UiO-66-NH2/TiO2/ZnO. For the identification of NC, scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET), Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD) techniques were used. Understanding the recovery mechanisms and performance behind the proposed combination is vital and beneficial when it comes to the ability to extract more amounts of oil economically. In the current research, various tests such as stability test, interfacial tension (IFT), contact angle and coreflooding were conducted. The major conclusions are listed as follows:
-
1.
It is necessary to perform interfacial tension, contact angle and coreflooding tests to minimize the oil-wetting nature of the rock.
-
2.
The stability test showed that SmW and 100 ppm NC solution has very good stability compared to other solutions.
-
3.
The effective smart water (SmW) + 100 ppm NC solution caused significant changes in the contact angle of the rock, these changes caused the wettability of the rock to change from 161º to 28º.
-
4.
The results showed that the SmW + 100 ppm NC solution has significantly reduced the IFT of water and oil.
-
5.
Coreflooding tests showed that oil recovery using smart water (SmW2Sulfate) + 100 ppm NC flooding is higher compared to smart water (SmW2 calcium) + 100 ppm NC flooding. In this regard, oil recovery through SmW(SmWSulfate) + 100 ppm NC is about 9.8% and oil recovery through SmW(SmW2calcium) + 100 ppm NC is 5.9%. In general, coreflooding tests provide a wider understanding for better reservoir development.
-
6.
The results showed that this new synthetic nanocomposite is able to create a very strong pressure gradient mechanism to separate oil from the rock surface.
-
7.
Ion analysis showed that the process of ion exchange and carbonate dissolution increased the tendency of oil to separate from the carbonate surface and also caused wettability alteration of rock (from oil-wet to water-wet) and increased oil recovery.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Ca2+ :
-
Calcium
- CR:
-
Carbonate rock
- FW:
-
Formation water
- IFT:
-
Interfacial tension
- NC:
-
Nanocomposite
- NCs:
-
Nanocomposites
- NF:
-
Nano-fluid
- NFs:
-
Nano-fluids
- NPs:
-
Nanoparticles
- OW:
-
Oil-wet
- SO4 2 − :
-
Sulfate
- SmW:
-
Smart water
- SmW2ca:
-
SmW2calcium
- SmW2S:
-
SmW2sulfate
- SmWs:
-
Smart waters
- TZ:
-
TiO2/ZnO
- UiO-66-NH2 :
-
UN
- UNTZ:
-
UiO66-NH2/TiO2/ZnO
- WA:
-
Wettability alteration
- WW:
-
Water-wet
- ζ:
-
Zeta
References
Ahmadi MA, Shadizadeh SR (2013) Implementation of a high performance surfactant for enhanced oil recovery from carbonate reservoirs. J Pet Sci Eng 110:66–73
Ahmadi Y, Mansouri M (2021) Using new synthesis zirconia-based nanocomposites for improving water alternative associated gas tests considering interfacial tension and contact angle measurements. Energy Fuels 35(20):16724–16734
Ahmadi Y, Mansouri M, Jafarbeigi E (2022a) Improving simultaneous water alternative associate gas tests in the presence of newly synthesized γ-Al2O3/ZnO/urea nano-composites: an experimental core flooding tests. ACS Omega 8(1):1443–1452
Ahmadi Y, Mohammadi M, Sedighi M (2022b) Introduction to chemical enhanced oil recovery. Chem Methods, pp 1–32.
Alghamdi A, Ayirala S, Alotaibi M, Alyousef A (2019) SmartWater synergy with surfactant chemicals: an electro-kinetic study. In: Paper SPE 209360 presented at the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, November (2019). https://doi.org/10.2118/197239-MS.
Ali H, Soleimani H, Yahya N, Khodapanah L, Sabet M, Demiral BMR, Hussain T, Adebayo LL (2020) Enhanced oil recovery by using electromagnetic-assisted nanofluids: a review. J Mol Liq 309:113095
Ali JA, Kolo K, Khaksar Manshad A, Stephen KD (2021) Emerging applications of TiO2/SiO2/poly(acrylamide) nanocomposites within the engineered water EOR in carbonate reservoirs. J Mol Liq 322:114943
Ayirala S, AlSofi A, AlYousef Z, Wang J, Alsaud MA, AlYousef A (2022a) SmartWater based synergistic technologies for enhanced oil recovery. Fuel 316:123264
Ayirala SC, AlSofi AM, AlYousef ZA, Wang J, Alsaud MOA, AlYousef AA (2022b) SmartWater based synergistic technologies: a next recovery frontier for enhanced oil recovery. In: Paper SPE 209360 presented at the SPE improved oil recovery conference, virtual, April (2022). https://doi.org/10.2118/209360-MS
Engeset B (2012) The potential of hydrophilic silica nanoparticles for EOR purposes: a literateur review and an experimental study. Master thesisDepartment of Petroleum Engineering and Applied Geophysics, Norwegian University of Science and Technology, Trondheim, Norway
Farhadi H, Rakhodaei M, Naserian M, Ayatollahi S, Fatemi M (2020) Experimental investigation on low salinity effect using dynamic interfacial properties during tertiary water flooding. EAGE Annual Conf Exhib 1:1–5
Franco CA, Zabala RD, Bahamon I, Forero A, Cortes FB (2021) Field applications of nanotechnology in the oil and gas industry: recent advances and perspectives. Energy Fuels 35(23):19266–19287
Ghalamizade Elyaderani SM, Jafari A, Razavinezhad J (2019) Experimental investigation of mechanisms in functionalized multiwalled carbon nanotube flooding for enhancing the recovery from heavy-oil reservoirs. In: Paper 194499. SPE J 24(6):2681–2694. https://doi.org/10.2118/194499-PA
Gharibshahi R, Jafari A, Haghtalab A, Karambeigi MS (2015) Application of CFD to evaluate the pore morphology effect on nanofluid flooding for enhanced oil recovery. RSC Adv 5(37):28938–28949
Hassan YM, Guan BH, Chuan LK, Khandaker MU, Sikiru S, Halilu A, Adam AA, Abdulkadir BA, Usman F (2022) Electromagnetically modifiedwettability and interfacial tension of hybrid ZnO/SiO2 nanofluids. Crystals 12(2):169
Hosseini S, Sabet M, Zeinolabedini-Hezave A, Ayoub MA, Elraies KA (2020) Effect of combination of cationic surfactant and salts on wettability alteration of carbonate rock. Energy Sources, Part A, pp 1–17
Jafarbeigi E, Ahmadi Y, Mansouri M, Ayatollahi S (2022a) Experimental core flooding investigation of new ZnO−γAl2O3 nanocomposites for enhanced oil recovery in carbonate reservoirs. ACS Omega 7(43):39107–39121
Jafarbeigi E, Ayatollahi S, Ahmadi Y, Mansouri M, Dehghani F (2022b) Identification of novel applications of chemical compounds to change the wettability of reservoir rock: a critical review. J Mol Liq 371:121059
Jafarbeigi E, Kamari E, Salimi F, Mohammadidoust A (2020a) Synthesis and modification of graphene nanofluid surface in terms of wettability alteration of carbonate reservoir rock. Energy Sources, Part A 1–15
Jafarbeigi E, Kamari E, Salimi F, Mohammadidoust A (2020b) Experimental study of the effects of a novel nanoparticle on enhanced oil recovery in carbonate porous media. J Pet Sci Eng 195:107602
Jafarbeigi E, Mansouri M, Talebian SH (2023) Effect of UiO-66-NH2/TiO2 nano-fluids on the IFT reduction and their use for wettability alteration of carbonate rocks. Mater Chem Phys 299:127496
Jafarbeigi E, Salimi F, Kamari E, Mansouri M (2021) Effects of modified graphene oxide (GO) nanofluid on wettability and IFT changes: Experimental study for EOR applications. Pet Sci 19(4):1779–1792
Jafarbeigi E, Mohammadidoust A, Ranjbar B (2022c) A review on applications of nanoparticles in the enhanced oil recovery in carbonate reservoirs. Pet Sci Technol 40(15):1811–1828
Karkare MM (2014) Choice of precursor not affecting the size of anatase TiO2 nanoparticles butaffecting morphology under broader view. Internat Nano Lett 4(8)
Khaksar-Manshad A, Ali JA, Haghighi OM, Sajadi SM, Keshavarz A (2022) Oil recovery aspects of ZnO/SiO2 nano-clay in carbonate reservoir. Fuel 307:121927
Kobayashi M, Juillerat F, Galletto P, Bowen P, Borkovec M (2005) Aggregation and charging of colloidal silica particles: Effect of particle size. Langmuir 21:5761–5769
Koleini MM, Badizad MH, Mahani H, Dastjerdi AM, Ayatollahi S, Ghazanfari MH (2021) Atomistic insight into salinity dependent preferential binding of polar aromatics to calcite/brine interface: implications to low salinity waterflooding. Sci Rep 11(1):1–17
Kumar N, Mandal A (2018a) Oil-in-water nanoemulsion stabilized by polymeric surfactant: characterization and properties evaluation for enhanced oil recovery. Eur Polym J 109:265–276
Kumar N, Mandal A (2018b) Thermodynamic and physicochemical properties evaluation for formation and characterization of oil-in-water nanoemulsion. J Mol Liq 266:147–159
Kumar S, Panigrahi P, Saw RK, Mandal A (2016) Interfacial interaction of cationic surfactants and its effect on wettability alteration of oil-wet carbonate rock. Energy Fuel 30:2846–2857
Li TT, Liu YM, Wang T, Wu YL, He YL, Yang R, Zheng SR (2018) Regulation of the surface area and surface charge property of MOFs by multivariate strategy: synthesis, characterization, selective dye adsorption and separation. Micropor Mesopor Mater 272:101–108
Lopez D, Zabala RD, Cardenas JC, Lopera SH, Riazi M, Franco CA, Cortes FB (2020) A novel design of silica-based completion nanofluids for heavy oil reservoirs. J Pet Sci Eng 194:107483
Mahani H, Keya AL, Berg S, Bartels WB, Nasralla R, Rossen WR (2015) Insights into the mechanism of wettability alteration by low-salinity-flooding (LSF) in carbonates. Energy Fuels 29(3):1352–1367
Mansouri M, Ahmadi Y, Jafarbeigi E (2022) Introducing a new method of using nanocomposites for preventing asphaltene aggregation during real static and dynamic natural depletion tests. En Energy Sources Part A 44(3):7499–7513
Mansouri M, Olya ME, Lotfi H, Nademi M (2017) Investigation of UV/TiO2-ZnO-Co photocatalitic degradation of azo dye (Reactive red 120) by response surface methodology. Sci. Study Res Chem Chem Eng Biotech Food Industry 18:153–165
Mofokeng SJ, Kumar V, Kroon RE, Ntwaeaborwa OM (2017) Structure and optical properties of Dy3 activated sol-gel ZnO-TiO2 nanocomposites. J Alloys Comp 711:121–131
Moradi S, Azar PA, Farshid SR, Khorrami SA, Givianrad MH (2012) Effect of activitieson characterization and photocatalytic activity of TiO2/ZnOnanocomposite prepared via sol-gelprocess. Int J Chem Eng, 215373.
Namaee-Ghasemi A, Ayatollahi S, Mahani H (2023) Insights into the Effects of pore structure, time scale, and injection scenarios on pore-filling sequence and oil recovery by low-salinity waterflooding using a mechanistic DLVO-based pore-scale model. In: Paper 214320. SPE J, pp 1–17. https://doi.org/10.2118/214320-PA
Nazari Moghaddam R, Bahramian A, Fakhroueian Z, Karimi A, Arya S (2015) Comparative study of using nanoparticles for enhanced oil recovery: wettability alteration of carbonate rocks. Energy Fuels 29(4):2111–2119
Nourinia A, Khaksar-Manshad A, Shadizadeh SR, Ali JA, Iglauer S, Keshavarz A, Mohammadi AH, Ali M (2022) Synergistic efficiency of zinc oxide/montmorillonite nanocomposites and a new derived saponin in liquid/liquid/solid interface-included systems: application in nanotechnology-assisted enhanced oil recovery. ACS Omega 7(29):24951–24972
Nowrouzi I, Khaksar-Manshad A, Mohammadi AH (2022) Effects of MgO, γ-Al2O3, and TiO2 nanoparticles at low concentrations on interfacial tension (IFT), rock wettability, and oil recovery by spontaneous imbibition in the process of smart nanofluid injection into carbonate reservoirs. ACS Omega 7(26):22161–22172
Pal N, Kumar N, Saw RK, Mandal A (2019) Gemini surfactant/polymer/silica stabilized oil-in-water nanoemulsions: design and physicochemical characterization for enhanced oil recovery. J Pet Sci Eng 183:106464
Rasooli-Manesh R, Kohnehpoushi M, Eskandari M, Fakhroueian Z, Abdollahi Nejand B (2017) Synthesis and evaluation of nano γ-Al2O3 with spherical, rod-shaped, and plate-like morphologies on enhanced heavy oil recovery. Mater Res Express 4(9):095025
RezaeiDoust A, Puntervold T, Strand S, Austad T (2009) Smart water as wettability modifier in carbonate and sandstone: a discussion of similarities/differences in the chemical mechanisms. Energy Fuels 23:4479–4485
Rostami P, Mehraban MF, Sharifi M, Dejam M, Ayatollahi S (2019) Effect of water salinity on oil/brine interfacial behaviour during low salinity waterflooding: a mechanistic study. Petroleum 5(4):367–374
Saw RK, Mandal A (2020) A mechanistic investigation of low salinity water flooding coupled with ion tuning for enhanced oil recovery. RSC Adv 10:l42570-42583
Saw RK, Pillai P, Mandal A (2022) Synergistic effect of low saline ion tuned sea water with ionic liquids for enhanced oil recovery from carbonate reservoirs. J Mol Liq 364:120011
Soleimani H, Hamza MF, Merican ZMA, Stephen KD (2022) Sol-gel synthesis of silicadioxide/organopolymer nanocomposite for potential applications in EOR nanotechnology. Towards intelligent systems modeling and simulation: with applications to energy, epidemiology and risk assessment, pp 357–365.
Soni BH, Deshpande MP, Bhatt SV, Garg N, Chaki SH (2013) Studies on ZnOnanorods synthesized by hydrothermal method and their characterization. J Nano Electr Phys 5:04077
Strauss I, Chakarova K, Mundstock A, Mihaylov M, Hadjiivanov K, Guschanski N, Caro Ju (2020) UiO-66 and UiO-66-NH2 based sensors: dielectric and FTIR investigations on the effect of CO2 adsorption. Micropor Mesopor Mater 302:110227
Suleimanov BA, Abbasov HF, Ismayilov RH (2022) Enhanced oil recovery with nanofluid injection. Pet Sci Technol, pp 1–18.
Suleimanov BA, Ismailov FS, Veliyev EF (2011) Nanofluid for enhanced oil recovery. J Pet Sci Eng 78(2):431–437
Taborda EA, Franco CA, Lopera SH, Castro RH, Maya GA, Idrobo EA, Cortes FB (2021) Effect of surface acidity of SiO2 nanoparticles on thermal stability of polymer solutions for application in EOR processes. J Pet Sci Eng 196:107802
Yang Y, Cheng J, Wang B, Guo Y, Dong X, Zhao J (2019) An amino-modified metal-organic framework (type UiO-66-NH2) loaded with cadmium(II) and lead(II) ions for simultaneous electrochemical immunosensing of triazophos and thiacloprid. Microchim Acta 186:101
Yang Y, Yao HF, Xi FG, Gao EQ (2014) Amino-functionalized Zr(IV) metal-organic framework as bifunctional acid-base catalyst for Knoevenagel condensation. J Mol Catal A Chem 390:198–205
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Mohsen Mansouri, Ehsan Jafarbeigi, Yaser Ahmadi and Seyyed Hossein Hosseini. The first draft of the manuscript was written by Mohsen Mansouri and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest with each other.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript. All authors are fully aware of this manuscript and have permission to submit the manuscript for possible publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mansouri, M., Jafarbeigi, E., Ahmadi, Y. et al. Experimental investigation of the effect of smart water and a novel synthetic nanocomposite on wettability alteration, interfacial tension reduction, and EOR. J Petrol Explor Prod Technol 13, 2251–2266 (2023). https://doi.org/10.1007/s13202-023-01676-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01676-5