Abstract
Asphaltenes behave like blood cholesterol in that they deposit on the walls of crude oil transportation pipes thereby narrowing the internal diameters, thus posing great dangers. This study was designed to remove asphaltenes from light crudes by solvent precipitation and to investigate the comparative performance of n-heptane (single solvent) and n-pentane/n-heptane (mixed solvent) in this regard. Each of three Nigerian crudes: Bonny Export, Bodo and Mogho crudes were first distilled at 350 °C to obtain the atmospheric residuum. Asphaltenes were precipitated from each residuum at different stirring times with single n-heptane and mixed n-pentane + n-heptane solvents. The precipitated asphaltenes were characterized with FTIR, UV–visible spectrophotometers while the maltenes were fractionated to obtain the various fractions. Results show that the asphaltenes were made up of saturated (cyclic aliphatic hydrocarbons) and unsaturated (substituted aromatic hydrocarbon). Also, aromatics to saturates ratio and resins to asphaltenes ratio was higher in Bonny Export and lower in Mogho crude, thus, indicating that Bonny Export has the lowest asphaltene precipitation risk while Mogho crude has the highest risk. The results also showed that resins stabilize asphaltenes in crude as addition of resins to the different crudes reduced the quantity of asphaltene precipitated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crude oil is a multi-component mixture consisting of naturally occurring hydrocarbons, together with organic compounds of sulphur, nitrogen and oxygen, as well as trace amounts of metallic porphyrins, such as those of vanadium, nickel and iron. The origin of crude oil can have a significant effect on its composition. As a result, crude oils widely vary in volatility, density, viscosity and color. Crude oil may also contain dissolved inorganic gases, such as nitrogen, carbondioxide, and hydrogen sulphide, at high pressure and temperature conditions.
The conventional methods of physical fractionation used in the petroleum industry are based on physico-chemical characteristics and include distillation, extraction with solvents, and adsorption by surface active substances. The fractionation by extraction with non-polar solvents of similar nature, but of different boiling points, is a separation analogous to fractional distillation which yields basically complex fractions, first by boiling point ranges and consequently by molecular weight ranges (Dehkisia et al. 2004; Hoiberg 1965; Suelves et al. 2003). The amount of fractions separated depends on the extent of dilution, temperature, and the nature of the solvents, as well as the equilibrium of the system (Hong and Watkinson 2004; Speight 1980).
Asphaltenes are important constituents in crude oil. They are usually defined as a fraction of crude oil soluble in aromatic solvents such as toluene or benzene and insoluble in paraffinic solvents such as n-pentane or n-heptane. Asphaltenes contribute significantly to the high viscosity and the coking tendency of heavy oils and bitumen. They cause solid deposits that obstruct flow in petroleum production systems (Auflem 2002). Asphaltene can self-associate and/or precipitate, but the self-association and precipitation is mediated by other solubility fractions particularly resins (Speight 1999). Hence, asphaltenes and resins have often been lumped together as residue in crude oil, causing reduction in crude oil production as they block the pores of reservoir rocks and can plug the wellbore tubing, flowlines, separators, pumps, tanks and other equipment and as a result cause barrier to the flow of oil (Leontaritis and Mansori 1987).
The separation of residues into two fractions asphaltenes and maltenes is brought about by means of low molecular weight paraffinic hydrocarbons, which have been recognized as possessing selective solvency for hydrocarbon systems and is known as solvent de-asphalting. The precipitation of insolubles from petroleum is known to follow a complex mechanism involving solubility equilibria, adsorption, and aggregation. Thus, the amounts and nature of insolubles precipitated by different hydrocarbon solvents will be different. Ethyl acetate has been used to eliminate both resins and asphaltenes from the oil in one step and thus improving the quality of de-asphalted oil (Catellanos et al. 1983).
The objective of this study was to investigate the effects of pure solvent, mixed solvent systems, addition of resin and variation in stirring time on asphaltene precipitation and also characterize the resulting asphaltenes precipitated.
Experimental
Sample source
Three Nigerian light crude oils namely: Bonny Export (49.91° API gravity) and Mogho (48.9° API gravity) from Rivers State and Bodo (36.95° API gravity) from Delta State were used in the study.
Reagents
n-Pentane (Sigma-Aldrich), n-heptane (FSA, England), dichloromethane, toluene, silica gel (BDH, England) and methanol (Fischer chemicals) were used in this study. All other reagents were of analytical grade.
Precipitation and purification of asphaltenes
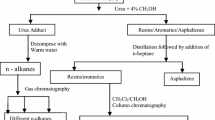
The three different crudes were distilled at 350 °C and the dead crude obtained was stored in a reagent bottle. 40 mL of n-heptane single solvent or n-pentane + n-heptane (1:1 volume ratio) mixed solvents was mixed with 1 mL of each of the crudes. The mixture was stirred for 20, 40, 60 and 80 min, respectively, and allowed to equilibrate for 48 h. After equilibration, the mixture was centrifuged for 30 min at 200 rpm and the resulting maltenes were decanted. The solid residue mainly precipitated asphaltenes was rinsed with 40 mL of the liquid precipitant until a clear solvent was obtained. The precipitated asphaltenes were dried in a vacuum oven at 80 °C to constant weight.
The precipitated asphaltenes were purified by dissolving each asphaltene in 10 mL toluene and adding 20 mL of n-alkanes to the filtrate so as to re-precipitate asphaltenes. The re-precipitated asphaltene was dried at 80 °C to constant weight. The melting point, UV (6405UV/Vis spectrophotometer, Jenway, England) and IR (FTIR spectrophotometer) spectra of the asphaltenes were carried out.
Fractionation of maltenes
The different components of the maltenes (saturates, aromatics and resins) were recovered using the SAR method. This involves packing a column with activated silica gel and adding 50 mL dichloromethane and 5 mL of maltenes. Addition of n-heptane elutes saturates, toluene elutes the aromatics while 1:1 dichloromethane and methanol elutes the resins.
Resin stabilizing effect on asphaltenes
40 mL of n-heptane was added to 1 mL of the different crudes and the resin extracted as described above were added to the different mixtures, respectively. The mixture was stirred for 80 min and extraction was done as reported in “Experimental” section.
Results and discussion
Tables 1 and 2 show the IR spectra of the crudes for the single and mixed solvents, respectively. The three crudes show characteristic frequencies at 3,056.31, 3,060, 3,080 that are due to C–H stretch for aromatic hydrocarbons. This is supported by absorptions at 733.94, 734.90, 736.83 that are due to substituted aromatic hydrocarbons. This confirms the same class of crude oil composition. This class of crude oil composition consists of fused benzene rings. However, absorptions at 2,933.83, 2,930.93, 2,929.97 and 2,931.9 are due to cyclic aliphatic hydrocarbons. This is supported by absorptions at 1,264.34, 1,271.17, 1,265.35, 1,276.92, 1,266.31 that are due to C–H bending. The IR reveals that asphaltenes fractions of the three crudes are made up of both saturated and unsaturated portions in the chemical structures.
Table 3 shows the UV–visible spectra of asphaltene fractions from the three crudes using n-heptane single solvent and n-pentane + n-heptane mixed solvent for 80 min stirring time. Each of the asphaltene fractions shows absorption maxima (Abs) in the visible region of the electromagnetic spectrum, indicating that the C7 and C5 + C7 asphaltenes are largely unsaturated. Absorptions at the following wavelengths: 388.8, 388.9, 387.2 and 388.6 nm which are supported by absorption at 418.2, 509.6, 510.0, 449.4, 482.6 and 432.8 nm suggest the same classes of crude oil composition which is the presence of fused benzene rings or polynuclear aromatics in asphaltenes which indicate unsaturated and highly conjugated systems. This implies the existence of chromophores in the asphaltene which result from conjugated double bonds involving aromatic hydrocarbons as supported by the infrared spectra. The UV–Visible absorption bands are similar to those shown elsewhere (Evodokimov and Losev 2007).
It is known that asphaltene precipitation depends on the stability of the asphaltenes and stability depends not only on the properties of the asphaltene fraction but on how good a solvent and the rest of the oil is for its asphaltenes. Figures 1 and 2 show that the percentage weight of asphaltenes from Bonny Export crude increases with increase in stirring time for both single and mixed solvents while Bodo and Mogho crude did not follow the same trend. The densities of their maltene (Table 4) increase with their corresponding asphaltene precipitate. Thus, it supposes that asphaltene precipitation depends on stirring time but due to the presence of other solubility parameters (saturates, aromatics and resins) which may not be present in the right ratios, Bodo and Mogho did not depend on stirring time. It may also be because the other solubility fractions in Bodo and Mogho crudes are not good solvents for their asphaltenes, therefore making their asphaltenes unstable and so precipitation in these crudes were more and did not depend on stirring time. It was also observed that Mogho crude precipitated more asphaltenes followed by Bodo and Bonny for both single and mixed solvent systems. This indicates that the amount and characteristics of the asphaltenes constituent in crude oils depend to a greater extent on the source of the crude (Speight 2004). It was also observed that for the three crudes, the amount of asphaltene precipitated increased on using mixed solvent (n-pentane + n-heptane) an observation explainable by the contribution of the shorter chain-length hydrocarbon (n-pentane). This agrees with fact that asphaltene precipitation increases with decrease in the carbon chain-length of the precipitating agent (Diallo et al. 2000).
Figure 3 shows the weight of heavy fractions obtained from n-heptane at 80 min stirring time. The ratio of aromatics to saturates was Bonny Export (0.313), Bodo (0.277) and Mogho (0.264) while the ratio of resins to asphaltenes was Bonny export (12.1), Bodo (2.180) and Mogho (2.000). Reports have it that a high ratio of resins to asphaltenes and aromatics to saturates is indicative of low asphaltene precipitation risk (Diallo et al. 2000). Heavier oil contains many of intermediate components that are good asphaltene solvents, whereas the light oil may consist largely of paraffinic materials. The three crudes show high values of saturates, indicating the presence of paraffinic material. Crude oils with higher densities also show higher cohesive energies and therefore lower the solubility in the crude oil. This study shows that the density and aromaticity of the crudes increase simultaneously as the asphaltene precipitates increase from Bonny export, Bodo and Mogho crudes. This shows that crude oil with higher aromaticity, saturates and density like in Bodo and Mogho crudes precipitate more asphaltene and are likely to be problematic and thus referred to as unstable crudes.
Figure 4 shows that the percentage weight of asphaltenes obtained when resins were added to the three crudes greatly reduced when compared to those without resins. This shows that resins stabilize asphaltenes in crude oil.
Conclusions
This study has shown that density and aromaticity of atmospheric residuum increases simultaneously with increase in asphaltenes. It has also shown that Mogho crude has the highest asphaltene precipitate and Bonny export the least, thus Mogho crude with the highest density, saturate, aromaticity and asphaltenes will likely be problematic. Also, it was established that there is a drastic reduction of asphaltene precipitate in Bonny, Bodo and Mogho crudes with additional resins extracted from the same crudes that resins from one crude oil solubilize asphaltenes from the same crude oil.
References
Auflem IH (2002) Influence of Asphaltene aggregation and pressure on crude oil emulsion stability. PhD thesis, Norwegian University of Science and Technology, Trondheim
Catellanos E, Hans I, Neumann J (1983) Thermal cracking, thermal hydrocracking and catalytic cracking of deasphalted oils. Fuel Sd Technol Int 11:1731–1758
Dehkisia S, Larachi F, Hornet E (2004) Catalytic (Mo) upgrading of Athabasca bitumen vacuum bottoms via two-step hydrocracking and enhancement of Mo-heavy oil interaction. Fuel 83:1323–1331
Diallo MS, Cagin T, Faulon JL, Goddard WA (2000) Asphaltenes and asphalts, 2. Developments in petroleum science. In: Yen TF, Chilingarian TF (eds) Thermodynamics properties of asphaltenes: a predictive approach based on computer assisted structure elucidation and atomistic simulation, vol 2. Elsevier Science Ltd, Los Angeles, pp 103–127
Evodokimov IN, Losev AP (2007) Potential of UV-visible absorption spectroscopy for characterising crude petroleum oils oil and gas business, pp 21–42
Hoiberg AJ (1965) Bituminous material, asphalts, tars and pitches. Interscience, New York
Hong E, Watkinson P (2004) A study of asphaltene solubility and precipitation. Fuel 83:1881–1887
Leontaritis KJ, Mansori GA (1987) Asphaltene deposition during oil production and processing: thermodynamic colloidal model, SPE 16258, SPE International Symposium on Oil Field Chemistry. San Antonio
Speight JG (1980) Chemistry and technology of petroleum. Marcel Dekker Inc, New York
Speight JG (1999) The chemistry and technology of petroluem. Marcel Dekker, New York
Speight HG (2004) Petroleum asphaltenes part 1: asphaltenes, resins and the structure of petroleum. Oil Gas Sci Tehcnol Rev 59:467–477
Suelves I, Islas CA, Millan M, Galmes C, Carter JF, Herod AA et al (2003) Chromotagraphic separations enabling the structural characterization of heavy petroleum residues. Fuel 82:1–14
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nwadinigwe, C.A., Anigbogu, I.V. & Ujam, O.T. Studies on precipitation performance of n-heptane and n-pentane/n-heptane on C7 and C5/C7 asphaltenes and maltenes from 350 °C atmospheric residuum of three Nigerian light crudes. J Petrol Explor Prod Technol 5, 403–407 (2015). https://doi.org/10.1007/s13202-014-0150-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-014-0150-x