Abstract
Global warming is affecting animal populations worldwide, through chronic temperature increases and an increase in the frequency of extreme heatwave events. Reservoirs are essential for water security. All watersheds with reservoirs are impacted by their construction. These artificial ecosystems controlled by humans change considerably the natural terrestrial and aquatic ecosystem and systems and their biodiversity. The rapid increase in population growth, urbanization, and industrialization are accompanied by an increase in river discharges, which increases the total amount of pollutants. HMs contamination in aquatic environments, as well as the subsequent absorption of HMs into the food chain by aquatic creatures and people, endangers public health. Multiple uses of reservoirs promote benefits in terms of economic development, income, and employment. HMs in water can be ingested directly by aquatic species like fish and can also be ingested indirectly through the food chain; thus, it is much more important and required to conduct frequent monitoring of the aquatic environment. As a result, this review summarizes knowledge about the effects of cascade dams on river water temperature and increases on the stress physiology of fishes, and adaptation to climate change is also needed to produce more fish without global warming.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The history of dam construction is rich and complex. The majority parts, dams are constructed to provide the aims of flood prevention, water management, power generation, and river navigation. The environmental consequences of such dams are considerable, although all need to be carefully investigated before being adequately categorized. Human engineering infrastructure, on which all survival depends, includes the building of large dams. There is a variety of advantages for human societies whenever rivers are dammed. Dams and reservoirs can collect water to be used afterward, allowing for a steady outflow and adequate inflow throughout the year. While utilizing natural resources to improve human life, it is important to keep environmental consequences in mind or under control. Dam construction provides challenges and opportunities as a part of integrated watershed management (Zhu et al. 2022). The hydrography and morphological evolution of rivers are influenced by changes in flow velocity, water quality, temperature, turbidity, particle material, or other physicochemical factors whenever dams are closed, and rivers are transformed into static reservoirs. The biogeochemical cycles of minerals are significantly disrupted by dams, which has consequences for wetland ecosystems, estuaries, submerged deltas, and the marine ecosystems downstream. Currently, increasingly serious marine biological and environmental problems are raising the need for effective and timely monitoring. Compared with traditional marine monitoring technologies, UAV remote sensing is becoming an important means for marine monitoring thanks to its flexibility, efficiency, and low cost, while still producing systematic data with high spatial and temporal resolutions (Yang et al. 2022). Excessive reactive N in terrestrial and marine ecosystems from large applications of fertilizer has impacted the balance of the global N cycle and contributed to numerous eco-environmental problems, such as widespread eutrophication, hypoxia expansion, and increased harmful algal blooms (Lin et al. 2021). The recent global warming, both in terms of temperature and precipitation, may also increase the harmful consequences of dam construction on the biological environment. Furthermore, the global industrial revolution has caused the dispersion of toxic compounds into the environment at a rate never seen before. Exposure to these pollutants can harm human health over the long term, notably via the consumption of plant-based foods and drinks, drinking water, or breathing polluted air (Nriagu 1990). The behavior of contaminants varies depending on how they are mixed with water. Natural self-purification processes destroy nonconservative materials, such as organics, certain inorganic, and countless microbes, resulting in a reduction in their concentration (Hao and Wang 2009). Metals may poison people and wildlife through hazardous industrial, municipality, and urban runoff. For the rise of trace metals, particularly heavy metals (HMs), in our rivers, urbanization, and industrialization have to be held responsible (Ali et al. 2021). Many hazardous chemical components, when discharged into the environment, accumulate in marine sediments of water bodies, where they may cause health problems. The development and existence of living organisms depend on a variety of nutrients (Ali et al. 2019a). However, despite their importance, metal nutrients have a restricted range of needs, and a severe imbalance in the proportions of the elements may cause exposure to high amounts of metals, which can cause fish mortality and significant illness in humans. Because of this, major, minor, hazardous, and trace components in biological and agricultural products are being investigated (Piechota et al. 2013). As a result, worldwide scientists are paying close attention to metal nutrients in fruit and vegetables, soils, rivers, and surrounding ecosystems, as well as the metal contamination pathways in the dietary food chain, because of these complications (Alengebawy et al. 2021). Metals and metalloids having a density of over 4 ± 1 g/cm3 are referred to as HMs. The HMs include over 50 elements, 17 of which have been very hazardous and challenging to the environment. These HMs are present naturally in the earth's crust, where they may be found in natural background quantities in a variety of environments including soils, rocks, sediments, waterways, and microorganisms (Duffus 2002). Since HMs cannot be degraded or eliminated, they remain in the environment in their unreactive state (Ghosh and Singh 2005). Because of technological advancements, the growth of companies on or along the banks of rivers has become one of the most significant sources of pollution, posing health risks to the people who consume tainted water and other associated foods (Jadia and Fulekar 2009). Therefore, this review summarizes knowledge about the effects of temperature increases on the stress physiology of fishes and provides a scientific basis for the protection of the ecological environment, the impacts of damming on flow and thermal regimes, cumulative impacts in cascade reservoirs, and adaptation to climate change is also needed to produce more fish without global warming.
Scientific route for selection of articles
The present study is based on the articles and reports accessed from various Web-based search engines such as Research Gate, Academia.edu, Google Scholar, Web of Science (WoS), and Scopus. For this review study, keeping the research topic in mind, four key terms such as (a) cascade reservoir, (b) global warming, (c) fish behavior, (d) river water temperature, (e) heavy metals, and (f) fish ecosystem were chosen. These keywords were searched in the Web-based search engines to find journal articles and reports published in the last 3 decades from 1990 to 2022. The search strategy and article selection processes are shown in Fig. 1. All the articles gathered from the search engines were initially screened for in-depth review concerning the key terms as defined in this study. Hence, this study reviewed those articles primarily focused on the effects of river temperature increases on the stress physiology of fishes, the impacts of damming on flow and thermal regimes, and cumulative impacts in cascade reservoirs, providing a scientific basis for the protection of the ecological environment, and adaptation to climate change is also needed to produce more fish without global warming.
Climate change threat to water species
A rise in water temperature increases summer anoxic conditions in deep waters of stratified lakes, thereby impacting aquatic biodiversity and egg viability of cold water species like yellow perch (Kabel et al. 2012). In watersheds, species richness is threatened by climate change, often accelerated by human impacts. These impacts are varying between north and south regions. Some endemic species are particularly vulnerable to environmental change, while others may be abundant within their preferred habitat and not endangered (Thuiller et al. 2006). Vegetation is variable in sensitivity, ranging from negative to positive impacts on agricultural species and forests (Gu et al. 2016). Potential species extinctions from climatic change can occur in the boundaries of species distributions at lower latitudes. This can occur when summer temperatures increase in streams and shallow unstratified lakes, and cold water refuges are no longer available. Also, climatic change has large effects on nearshore biotic assemblages. With declining water levels, lakes might become separated from their bordering wetlands, and this could impact some species (Leibowitz 2003). Johnstone et al. (2016) observe that interactions between climate change and disturbance regimes may lead to rapid changes in vegetation composition and structure. These interactions can be from the early or late arrival of rains in seasonal areas of the tropics (Studds and Marra 2007). Climate change can also impact forests and macchia species cover, through reduced humidity, making it vulnerable to wildfires and further reducing the vegetation cover. This in turn accelerates land degradation in arid and semiarid watersheds (Lal 2012). In water bodies, climate-induced water-level fluctuations can negatively affect the structure of fish habitats like reed beds (Jeppesen et al. 2015), and the behavior and distribution of fish (Rilov et al. 2007). This can also change the physical parameters that influence hypoxia, resulting in an overall reduction in the fish stock, and changes in their community composition, abundance, and health (Gebrekiros 2016). Climate-induced droughts reduce the survival and reproduction rate of fish, promote emigration to other areas, and extinctions during severe droughts (Rogosch et al. 2019). Slow-moving, shallow waters are especially vulnerable to temperature fluctuations. Warmer waters can increase fungal, viral, and bacterial infections in fish, increase invasive species, and reduce dissolved oxygen, which results in changes to the regional distribution of taxa. Several macroinvertebrates are sensitive to differences of only 3 °C of mean summer temperature (Dallas and Rivers-Moore 2012). The extension of dry periods resulting from climatic change is a critical factor influencing watershed ecosystems. Dry periods that are longer than the regeneration times of the biota reduce their efficiency to survive and adapt to new conditions. Datry (2012) observe that the total invertebrate richness decreased linearly, while the invertebrate density decreased exponentially with the increasing duration of a dry period. The amount and quality of fish refugia in a given summer are dependent on the rainfall during preceding years (Magalhaes et al. 2007). Changes in runoff and water temperature change the riparian vegetation and influence macrophyte and algal growth (Ghermandi et al. 2009). Lower water levels and increased nutrient availability can lead to an increase in riparian plant biomass. Climate change impacts water temperature, flow regime, channel morphology, and sedimentation, which can be major factors in influencing invertebrates and fish in rivers (Soomro et al. 2023). The pattern of macrophyte abundance responds quickly to water-level and water quality changes and is a reliable indicator of stress (Manzoor et al. 2021). The effects of water-level changes on macrophytes are known in cases of reservoir floodplains and shallow lakes (Bakker and Hilt 2016), but less information is available for natural lakes and watersheds (Soomro et al. 2022). Low water levels also affect macrophyte abundance and patterns (Leira and Cantonati 2008). Interactions between climate change and lake biota are complex and species-specific and vary among ecosystems (Blois et al. 2013). Climate change affects species diversity and composition through the biological invasion of non-native species that expand in geographical range as temperatures warm (Broennimann et al. 2007). Climate change can increase vulnerability to wildfires, especially for slow-moving species (Lucas et al. 2007). Besides these, climate change can lead to irreversible impacts on plant and animal species. Approximately 20–30% of plant and animal species assessed so far are likely to be at increased risk of extinction for increases in global average temperature by 1.5–2.5 °C (Wu et al. 2010). In general, climate change will alter the ecohydrological conditions of lakes, streams, and rivers in most of the regions. In lakes, hydrological changes are expressed in terms of more dynamic fluctuations as well as overall changes in water level and their impact on eutrophication (Quan et al. 2022). These changes have direct impacts on aquatic and terrestrial biota.
Atmospheric regime of rivers
There are several factors to consider when assessing river temperatures, although they can be categorized into four broad categories: air content, geography, river flow, and river bed. The heat exchange mechanisms, including phase changes, which arise at the water's surface, were also entirely defined by atmospheric conditions, making them among the most crucial factors. Physical and biogeochemical heterogeneity dramatically impacts fluid flow and reactive solute transport behaviors in geological formations across scales (Zhang et al. 2021). Due to its impact on the regional weather, terrain, or geographic location situation is also essential. The thermal function is mainly affected by stream flow, which in turn is primarily influenced by river hydraulics; inflows and outflows, water quantity, and river bed thermal conversations or other formations of water integrating provide a cooling effect.
Spatial and temporal variability
Each of those reasons influences the overall temperature of river systems. For example, it is generally observed that the daily average water temperature increases even though moves downstream. The temperature of the water is typically identical to that of the surrounding groundwater (Kurylyk et al. 2019) and continues to expand as altitude and stream order increase. The rise in water temperature is massively greater for small streams and rivers than it is for larger ones, indicating that it is not a constant process. Large-scale changes can be observed; however, below the extreme where tributaries encounter, there is small-scale variation (Collischonn et al. 2007), along with bodies of water in which water seeps out (Rosenberry and Morin 2004) or at habitat scales (Mayor et al. 2009). The type of river also can influence how warm or cold it is. For instance, Ashmore and Sauks (2006) revealed that small, superficial channels in braided rivers that are extremely flexible to meteorological conditions could induce the water temperature to be extremely high. On the temporal scale, the water temperature varies, following both a diel and an annual cycle. Because of daily and seasonal changes, the temperature of the water is lower in the early hours (at sunrise) and high between both the midafternoon and the early evening hours. Daily variations are also normally minimal for colder watershed tributaries and increase over large tributaries because they become less controlled by groundwater and much more exposed to meteorological conditions.
River heat exchange processes
The regional and temporal variability of the temperature of the water is often determined by the aspects mentioned earlier; however, while modeling the river ecosystem, it's also important to consider either hydraulic forces or heat transfer mechanisms. Long-range precipitation forecasting is crucial for flooding control and water resources management. At least in sections that continue to flow tributaries, thermal effluent, and water extractions are negligible, and heat exchange transpires at the air/water surface and the streambed/water boundary. Numerous different factors, such as rain and turbulence, can be taken into account, but their impacts are generally not as big as those of the above factors. Friction can be essential, particularly in the autumn and winter months according to some research findings (Caissie 2006). Recently, a variety of machine learning models for river water temperature forecasting and precipitation were used worldwide (i.e., step-wise linear regression, random forest, XGBoost, feed-forward neural networks, recurrent neural networks). Liu et al. (2020) introduced a runoff generation framework for semiarid and semi-humid watersheds. In prior studies, the algorithm for every kind of energy at the air–water interface, precipitation was presented (Liyana-Arachchi et al. 2014; Müller et al. 2021; Wu et al. 2015) and therefore is easy to figure out using meteorological data from the meteorological station. Such energy components are strongly connected to river thermal processes and the physical processes that change the temperature of river water. For example, Research indicates that solar radiation constitutes the most significant component of the total energy flux, indicated by net long-wave emission and evaporation heat transfer (Sinokrot and Stefan 1993). Although convective heat exchange comprises a relatively small portion of the total energy flux, it is not in any respect negligible. Such flows have been evaluated in studies to focus exclusively on river temperatures. Notably, showed that (Webb and Zhang 1997) heat loss and gain in the Rivers are controlled by radiation emitted. Analyses such as these emphasized the importance of radiation and, through extension, riparian vegetation, the latter of which protects streams from excessive heat.
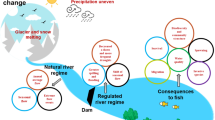
Consequences of damming on river flow and thermal regimes
Dam hydrologic alterations
Rivers are essential to aquatic biota and ecosystem functions because they perform a key role in the global water and biogeochemical system (Sabater 2008). Consequently, increasing dam construction has a profound effect on river flow dynamics (Ali et al. 2019b; Tang et al. 2016). In certain situations, dams are used to control water levels in rivers by reducing the high flows, offsetting low values, or storing and diverting the water downstream. Thus, the downstream river's flow regimes can be altered seasonally so water flow maxima were mitigated and dry-season low flows are significantly increased (Mezger et al. 2021). Dams can alter river flows over time, which has significant effects on river discharge, habitat, and ecological processes (Lehner et al. 2011). To construct hydropower sustainably and to effectively maintain reservoirs, an understanding of river flow patterns is required. Dams have changed flow regimes in various basins, as explored in recent research over the recent decade (Zhang et al. 2009). Hydrological investigations over a long period (Dimitriadis et al. 2021) and techniques for composite modeling (Willems and Vrac 2011) are frequently used to assess the effects of dam-induced variations to flow. In a basin, the consequences of single or cascading dams on flow trends at the regional scale are widely known to exist (Poff et al. 2007; Pringle 2001), in contrast to the challenges caused by climate change (Boulange et al. 2021). By evaluating the pre-impoundment time toward the post-impoundment time, it is clear that dams change and homogenize regional flow regimes (Poff et al. 2007). Seasonal hydrological sensitivity to dam discharge (Lajoie et al. 2007) and basin- or world-scale changes were gradual due to the effects of climate change (Gorczyc et al. 2017). The fundamental correlation between flow changes and river temperature regimes or water quality is an important and challenging issue.
Dam-induced changes in river thermal regime
Water quality and the migration of aquatic species both are influenced by the temperature regimes of rivers, which has a significant effect on the overall health of aquatic life (Huang and Izumiya 2003). The temperature regime of rivers can be drastically altered by the construction of dams (Magilligan and Nislow 2005), specifically in stratified dams, where the vertical density gradient creates a barrier preventing the combining of the water column (Omofunmi et al. 2017) and it regulates the vertical distribution of temperature and thermodynamic structure (Liu et al. 2019). Additionally, the discharge water is much warmer than the river's natural water (Nikora et al. 1994) and drastically impacts ecological environments. Therefore, it is crucial to study the mechanisms of temperature changes in reservoirs and the temperature dynamics of rivers. The reservoir's stratification behavior and the depth of preferential withdrawal simultaneously play significant roles in determining the magnitude of the change (Winton et al. 2019). By altering and diminishing the defined synchronized response of interacting air and water temperature, dams also can profoundly change a river's thermal regimes (Flanagan et al. 2015). Furthermore, variations in downstream river water temperature can also be caused by a decrease or fluctuation in flow (Bakken et al. 2016). Understanding the effects of stratification and reservoir operation on discharge is essential to downstream river ecology due to the importance of water temperature for biotic activities.
Cascade reservoirs effects on the environment
Special operations of cascade reservoirs have become increasingly prevalent as the number of dams around the world continues to increase. Downstream reservoir hydrodynamics in a cascade reservoir system is influenced not just by volume, geography, and operation of the upstream reservoirs, but also by water discharged from the upstream reservoirs, due to differences in water flow rate. The cumulative effects of several dams on the natural environment can be greater than the effects of any one dam (Cooper et al. 2017). Cascade hydroelectric reservoirs have many effects on the environment, resulting in an accumulation of spatial and temporal effects on ecosystems (Cardinale et al. 2004). However, due to interference, the superposition of impacts is not a simple addition. Either positive or negative consequences have occurred, based on how the dams are reversed (Zanjani et al. 2016). It reveals that cumulative effects from several dams can be complicated and unpredictable. For instance, the operational regulations, the size of the reservoir, along with the actual number and location of the dams, all have a role in the hydrological effects (Nathan et al. 2005; Quan et al. 2022). According to the limited data available, when cascade reservoirs are operated together, the cumulative effect increases (Huang et al. 2018). However, in particular cases, the processes of how alterations distribute and ultimately accumulate along the cascade remain unclear. This could prohibit us from having a complete understanding of how dams in a system of cascading reservoirs affect the environment.
Recent case study on the world's largest dam (TGD)
The great Yangtze river TGD
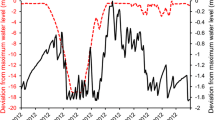
The TGD, being the largest hydraulic engineering project in the world, has been the subject of significant interest in the public and scientific research since prior construction started. In recognition of the issues which occurred whenever the Gezhouba Dam was built without adequate viability investigations, the Chinese government has become more careful with the strategy for TGD development. It demanded on a development plan as well as an EIR be completed before any construction on the TGD was started. This study was extensive and demanding since it involved gathering specialists and academics from various domains to discuss the potential costs and benefits of the TGD (Zhang 2015). An extensive research report on the ecology of water systems (lakes, rivers, etc.), including sedimentation and scour in river channels, aquatic biodiversity, and other related topics, was eventually accepted. Several academics, after the TGD's completion, Yan et al. (2021a) used trend analysis and modeling studies to forecast future effects of the TGD under several scenarios by comparing runoff and sediment discharge levels before and after impoundment (Mei et al. 2018). In 2003, the first TGD impoundment was built, bringing the water level to 139 m (Guo et al. 2012). Since the second impoundment began in 2006, the water level has risen to a whopping 156 m (Khurram et al. 2023). The full impoundment was completed in 2008, bringing the water level to 172.8 m and maximizing the project's flood control capability; the highest water level on record was reached in 2010 at 175 m (Li et al. 2021). As the flood season of June–September begins, the recommendations prescribe for the reservoir level to be lowered below the flood control level of 145 m in early June (Zheng et al. 2021). During this period, the rate at which water is released from the dam rises dramatically. While the dam is kept at a low level throughout the flood season, the water level is permitted to rise when the flow into the dam is relatively large to reduce the peak of the flood as it travels downstream. The flood waters recede to a level of 145 m after the storm has disappeared. From the beginning of October onward, the TGD will be recharging its reservoir, resulting in a reduced rate of discharge. Ultimately, the dam is operated for electricity generation between late November and May of the following year, and so the releases are often larger than they would be under natural conditions; during such a time the water level must be regulated above 155 m. The Map of Yangtze River (TGD) (Fig. 2)
Risk of dam effects on regional ecosystem
The damming of rivers has been observed to have adverse effects on ecosystems and other environmental elements in the surrounding area (Fu et al. 2010). In the case of the TGD, Zhang and Lou (2011) reported that the reservoir's filling has disturbed the physical habitat of aquatic life, including numerous endangered species and economically valuable fish. The loss of spawning sites due to dam construction is the primary cause of species population reductions. Similarly, hydrologic regimes are crucial in shaping the variety of life species available in a river system (Beechie et al. 2006; Wan et al. 2016) and a decrease in fish diversity has been recorded as a result of the TGD operation's alteration of the hydrologic regime. In China, dissolved organic matter (DOM) is an abundant and mobile part of the aquatic environment and plays important roles in aquatic biogeochemical cycles and the global carbon cycle. Recently, eutrophication has become an important environmental issue in global lakes, but how eutrophication drives changes in the molecular composition of DOM along trophic gradients remains poorly understood (Liu et al. 2022). Spawning of carp species requires sufficient water velocity to enable the eggs can float. Hence, sufficient increases in daily discharge and a minimum instream flow are required during the breeding season. In addition, New and Xie (2008) propose that dam management tries to mimic the normal dynamics of river flow as much as possible to reduce the negative effects on downstream ecosystems. Reservoir operations may need to be modified to enable adequate water-level changes to maintain areas that provide a habitat favorable for the spawning of carp (Wang et al. 2013) and TGD partially implemented. However, continued downstream channel degradation may counterbalance the ecological benefits of raising Yangtze levels in winter/fall and exacerbate the reduction in downstream lake inundation areas/levels (Wang et al. 2014). The TGD's reduced and streamlined Yangtze River geomorphology is expected to damage riparian ecology (Xu and Milliman 2009), producing geographically shorter, less diversified riparian ecosystems than pre-dam. The ability of rivers to filter out pollutants and eliminate toxins is likely to decrease as a result of TGD operation. The consequences of dispersion are mitigated by decreasing the height of the flood peak during the rainy season, which, in combination with the severe low levels, tends to further damage the quality of the river's water. There are many causes of the deteriorating water quality in the Yangtze, but two of the more prominent mammals are the river mammal and the finless porpoise. It’s because toxins are often adsorbed to the sediment particles, and most of these sediments are generally deposited upstream of the dam due to the interruption of the TGD. The Chinese government has authorized a sweeping initiative to monitor the Yangtze and the Three Gorges reservoir area for indications of contamination (Ranjan 2018). Dams not only have negative effects on the environment and biodiversity, but they can also have an effect on human health by influencing the ecology of waterborne and parasitic diseases like schistosomiasis (Zhou et al. 2016), not even the Three Gorges Dam can be an exception. Three Gorges Dam could cause schistosomiasis infections by changing the ecosystem in the Three Gorges and downstream areas (Zhu et al. 2008). They also hypothesized that the transmission of schistosomiasis in the Three Gorges and the lower Yangtze areas might be significantly affected by significant changes in population and agricultural practices (Shen et al. 2014). Since floods are employed to regulate snail populations, the decline in flood amplitude and frequency may be aggravating this condition (Yan et al. 2021b). In addition, the report by McManus et al. (2010) indicates that decreased snail concentrations downstream from the dam may help reduce schistosomiasis transmission. With lower and more constant water levels, snail populations may decrease, but infected snails and human infection may increase due to the colocation of buffalo grazing regions, snail habitat, and human activities.
Sources of HMs and their effects on the environment
The HMs exist naturally as components of the earth's crust but are also released because of human activity. As a result, they make up persistent environmental pollutants since they cannot be degraded or eliminated hence, they are often regarded as one of the most significant contributors to water contamination (Bradl 2005). HMs are released in both fundamental and combination (organic and inorganic) forms, and they are toxic to humans. It is predominantly got from rocks, modified soil, precipitation, and river systems that the availability of metals in the environment is observed (Pujari and Kapoor 2021). Because of various human activities such as transportation, building, manufacturing, fossil fuel burning, and pollution, soils may get polluted with metals in varying degrees. As a result, soils may get polluted by HMs such as Pb, Cr, and Hg, ranging from mild to severe levels of contamination. As a result, soils are most likely to offer some risk to garden foods and human health (Bradl 2005). According to scientists, the long-term deposition of these contaminants may diminish soil holding capacity and induce soil and groundwater pollution. Every year, the amount of trace metal pollution increases, posing a major threat to human health and a significant threat to the environment. Besides past and current mining areas, production lines and smelters, combustion by-products, and transportation are also examples of anthropogenic sources of emissions (Masindi and Muedi 2018). The sources of HMs differ from one location to the next. These include sewage and stormwater discharges, landfills and cemeteries, incinerators, crematoria, and motor vehicles, while a variety of human activities such as electroplating, smelting, dentists, research laboratories, timber retention, drum reconditioning, disposal storage and treatment, metal treatment, sheep and livestock dips, scrap metal yards, tanning, petrochemical plants, production and the use of capacitors, mercury lamps, thermometers, and utensils. HMs may be found in rocks as ores in a variety of chemical states, from which they can be extracted and refined into minerals (Alloway 2013). HMs ores include sulfides, such as Fe, As, Pb, Zn, Co, Cu, and Na, as well as oxides, such as Se, aluminum (Al), manganese (Mn), gold (Au), and antimony (Sb). Moreover, the Fe, Cu, and Co are extracted as both sulfide and oxide ores. By pedogenic processes, erosion underlying bedrock with just marginally higher metal concentrations may result in an accumulation of these metals (Masindi and Muedi 2018). As a result, soils, where increased metal concentrations in the bedrock are not known to exist, may also have naturally elevated levels of HMs. For the most part, powerful acid-generating processes in nature include the exposure of metal sulfides that are richer in HMs to the surrounding atmosphere, which results in oxidation and the creation of acid and/or HMs-rich water. Aside from that, weathering of coalmine waste may cause the formation of alkali compounds, HMs, as well as sediments (Sankhla et al. 2016). Acid sewage, alkaline molecules, HMs, and sediment derived from mine waste and leached into groundwater or wiped away by rainfall can cause pollution in streams, rivers, and ponds. HMs in wastewater have become a serious environmental issue in recent years because they pose a substantial danger to ecosystems and public health, even at low concentrations. Because of its adaptability, accumulation, non-biodegradability, and persistence, HMs pollution is a huge environmental burden. The HMs effluent from businesses such as paper industries, insecticides, tanneries, metal plating industries, and mining activities is toxic or damaging to human physiology and other biological systems. They can be reprocessed into less toxic forms. Toxic metals are hard to remove from wastewater because they might persist in chemical or mixed forms. These metals are vital for life, but they may also cause irreversible damage to the human body if consumed in high quantities (Pujari and Kapoor 2021). Aquatic life is wiped out by HMs in open waterways, which also causes oxygen depletion and algae blooms. HMs discharged into rivers are transformed into hydrated ions, which are much more poisonous than the metal atoms that originally contained them. The enzymatic mechanism is disrupted and absorption is sped up by these hydrated ions. HMs must thus be removed to reduce public danger (Sankhla et al. 2016). The WHO has defined a maximum permissible discharge threshold for HMs to reduce water pollution. To make matters worse for both humans and the ecosystem, the released wastewater includes much more HMs than is permitted (Bradl 2005). Over the last two centuries, rapid industrialization and sped-up global expansion have significantly boosted the pace at which HMs are discharged into the environment. The upshot is that many freshwater bodies are being severely degraded or destroyed (Masindi and Muedi 2018). A natural lake on the upstream Mississippi River (USA) revealed historical patterns in HMs usage and outflow in the watershed, according to the scientist. The lake, Lake Pepin is an example of this. HMs loadings in the river and aggregation in the lake have been attributed to both dispersed and point sources of contamination. HMs accumulated in Lake Pepin before Europeans, and most of the trace metals originated from dispersed natural sediment sources over the whole watershed. Later, as human activity in the watershed increased, urban and industrial wastes polluted the river and lake, contaminating them with HMs. Large levels of HMs have been discovered in the sediments and fish of India's enormous Ganges River system (Balogh et al. 2009).
HMs in river water and sediments
It has been shown that tiny metals are harmful such as Pb, Cr, and As from the land to the river. Several minor river banks and lakes, which are major conduits for the movement of metals and trace elements, may become substantial pollution sources (Verma and Dwivedi 2013). The behavior of HMs in water has been hypothesized to be a function of the sediments on which it is deposited, the suspended solids present, and the water's chemical makeup, according to further research. Scientists found that trace metals experience many changes in their speciation because of a variety of processes, including dissolution, precipitation, sorption, and complexation, all of which impact their behavior and bioavailability (Kar et al. 2008). As shown by recent research the deposition and dispersion of hydrocarbons, trace metals, and chlorinated molecules in soil, water, and the environment are growing at an alarming rate, causing deposition and sedimentation in water reservoirs, as well as adverse effects on aquatic organisms and seaweeds (Biney et al. 1994). A major issue in urban river basins is HMs contamination, which may be caused both by natural processes and human activities. Smelting, waste disposal, weathering, and the discharge of agricultural, urban/industrial, and municipal wastes may all contribute to metal contamination in the environment (Garai et al. 2021). HMs are environmental pollutants that are both stable and persistent in coastal sediments. The rising pollution of estuaries including harbors from a variety of human causes has become more concerning in recent years. In many cases, sediments serve as the best sink for toxins, and as a consequence, they represent the greatest threat to aquatic life when they are used as a source of pollution (Khayatzadeh and Abbasi 2010). Metal speciation in nearshore water sediments from Singapore was investigated by researchers who revealed that sediments are the primary repository and supply of HMs in the marine environment and that they play a significant role in the storage and transport of potentially hazardous metals. Several mechanisms have been identified as contributing to the buildup of pollutants in harbor sediments. Hydrodynamic energy is minimized in the design of the harbor; industrial actions (ship repairs and traffic; accidental spills; loading and unloading; and loading and unloading); agricultural (wastewater) activities; and urban (wastewater) actions are all potential sources of HMs pollution in the marine environment (Pujari and Kapoor 2021). HMs accumulation in marine sediment is caused by the highly dynamic existence of the marine environment, which allows for the rapid assimilation of pollutants into sediments through procedures such as oxidation, deterioration, dispersion, dilution, and ocean currents. HMs accumulation in marine sediment is also caused by the presence of HMs in the water column. HMs phyto-availability is determined by the features of the sediment, the type of the metal species, the interaction with the sediment matrix, and the length of time the metal is in contact with the surface binding. Marine organisms' capacity to metabolize HMs is linked to sediment qualities such as pH, organic matter concentration and type, and finally moisture. In general, population growth, unplanned industrialization, urbanization, exploration, exploitation of natural resources, and the introduction of new modern agricultural practices are the primary contributory factors to the presence of HMs in marine sediments (Fu and Wang 2011). In particular, rapid unplanned industrialization, urbanization, exploration, and exploitation of natural resources, and the introduction of new modern agricultural practices are the primary contributory factors to the presence of HMs in marine sediments (Ali et al. 2019a, b). Natural sources of HMs, such as leaching and rock weathering, are typical of little relevance when compared to human activities. HMs have been studied extensively in rivers and lakes. The HMs contamination and changes in environmental characteristics as well as the accumulation of HMs in fish muscles were also studied by researchers (Nriagu 1990). There will be changes in microbial communities because of HMs exposure since microbes are the most susceptible species to environmental changes. There is a broad tolerance for HMs contamination among microorganisms. HMs introduced into aquatic systems bind to particulate matter, which will ultimately settle and form part of the sedimentary record (Khayatzadeh and Abbasi 2010). The most significant place for metals and other contaminants to accumulate in aquatic ecosystems is surface sediment. Other aquatic species, such as macrophytes with deep root systems, may take up sediment-bound contaminants for removal. Environmental degradation caused by metals may occur even if water quality standards are not exceeded, but species in or near sediments are negatively impacted since a significant portion of the trace metals released into the aquatic ecosystem is ultimately linked to the bottom sediments (Tchounwou et al. 2012). Micro-pollutants, such as metals, which are often present in rivers, may alter the structure of diatom communities. HMs may have passed up the food chain by aquatic organisms that have collected them. Carnivores on the food web, such as humans, acquire most of their HMs load from the aquatic environment via their diet, particularly in areas where fish are prevalent, creating the potential for significant bio-magnifications. A variety of contaminants, including HMs, in aquatic systems, may cause a rise in the generation of ROS which can be harmful to fish and other aquatic creatures (Bhat et al. 2019). Fish is a product that has the potential to cause public health problems because it may be polluted with a variety of environmentally persistent pollutants, including HMs. Concern has been raised about the intake of fish that has high amounts of HMs in it, since long-term exposure to HMs may cause health concerns (Garai et al. 2021). As a pollutant, Hg is one of the most significant, both because of its impact on marine creatures and because it poses a potential threat to human health. Methylmercury is a harmful chemical compound of mercury that is generated in aquatic sediments because of bacterial methylation of organic mercury. Methylmercury accounts for virtually all the mercury found in fish muscles. Metallic ions are transported through the bloodstream of fishes, where they are frequently coupled to proteins to facilitate transport (Vajargah 2021). Metals are introduced into touch with the tissues and organs of the fish, and as a result, various amounts of metal accumulate in different tissues of the fish. There are five methods via which a contaminant might enter a fish. These pathways include food, non-food particles, gills, oral water ingestion, and the skin. Once the pollutants have been absorbed, they are carried by circulation either to a storage site (in this case, bone) or to the liver for modification and storage before being excreted by the body (Fatima et al. 2020). If the pollutants are changed by the liver, they may indeed be kept there or expelled in the bile, transported back into the bloodstream for probable excretion through the gills and kidneys, or stored in fat, which is an additional hepatic tissue. It has been frequently used to assess the ecological consequences of metal pollution in streams because benthic macro-invertebrate communities comprise species with a broad range of sensitivity to pollutants (Vajargah 2021). Because they are a crucial connection among primary producers and higher trophic levels in lotic food webs, they are also important in lotic ecosystems, where they regulate organic material decomposition and nutrient cycling, among other things. However, the influence of HMs on macroinvertebrates has still not been studied in terms of their nutritional value for fish, although invertebrates are a key food source for many species of moving-water fish, including many species of catfish. It is particularly vital to assess the impacts of HMs pollution on drift-prone macroinvertebrates, which are the food source for the majority of economically and recreationally significant salmonid species (Fatima et al. 2020).
Different HMs and their hazardous effects
The HMs such as arsenic (As), mercury (Hg), chromium (Cr), zinc (Zn), lead (Pb), selenium (Se), sodium (Na), cadmium (Cd), iron (Fe), cobalt (Co), and copper (Cu) are among the metals that contribute to contamination of water (Singh et al. 2011). Few HMs such as Fe, Zn, and Se are of biological significance to humans, and daily medical and dietary limits for these metals have been prescribed. However, others (such as As, Pb, Cr, and methylation forms of Hg) have been found to have no known biological significance in mammalian biochemistry and physiology, and their ingestion, even in scant amounts, may be harmful (Singh et al. 2011) (Fig. 3). Water habitats are severely impacted even at low concentrations of HMs, and there is no natural deterioration because of the pollution. Because it inhibits the microbial activity that were existing in the waste streams, it is considered environmentally friendly (Kahlon et al. 2018). Therefore, experts are currently concentrating their efforts on the removal of HMs from waste effluent, which is becoming more important. Many regulatory agencies across the globe have developed permitted limits for HMs discharge in wastewater because of these health consequences. Because wastewater includes crucial effects, researchers have focused on improving treatment methods (Masindi and Muedi 2018). The following section has information about some HMs and their properties.
Arsenic
In the Earth's crust, the Ar is ranked 21st among the elements in abundance, and it may be found in various forms in over 240 distinct elements in the environment, the most frequent of which are sulfides (also known as sulfosalts), and arsenates (also known as arsenites) (Mandal and Suzuki 2002). It may be found in saltwater 2–4 parts per billion (ppb) and rivers 0.5–2 ppb. Freshwater and seaweed algae contain approximately 1–250 parts per million (ppm) of As, while the freshwater macrophytes contain 2–1450 ppm, marine mollusks contain 1–70 ppm, marine crustaceans contain 0.5–69 ppm and fishes contain 0.2–320 ppm of arsenic (all values are based on dry mass) (Graeme and Pollack Jr 1998). The intake of polluted drinking water is the most common cause of As-related sickness in the USA. Because the indications of As poisoning match with cholera, it was used as a poison in the olden days, and the purposeful component was obscured as a result of this similarity (Alam et al. 2002). Recent research suggests that the toxicity of different As compounds varies. For instance, monomethyl arsenic acid and inorganic arsenide also have a greater toxicity level than arsenic choline, which has a lower toxicity level. The acute toxicity of inorganic As compounds is often greater than the acute toxicity of organic As compounds (Carter et al. 2003). The presence of As in the localized groundwater is already being reported from an increasing number of nations, and it is expected that many more examples will be identified shortly. Until lately, As was not generally included in the set of elements commonly examined by water testing laboratories, and as a result, there are likely to be numerous As-rich sources that have yet to be discovered (Gupta et al. 2017). As a result of the proposed adjustment of the As drinking water standard in several nations, the scenario in many countries has been re-evaluated. Because of the recently discovered widespread As pollution of alluvial water supplies in various rivers, it is imperative to conduct a quick evaluation of the status of alluvial aquifers across the globe (Smedley 2003).
Copper
Cu is regarded to be a very hazardous metal when present in large concentrations. It is a necessary element required by humans, and it plays an important part in enzyme manufacturing, bone growth, and the formation of cells and tissues (Alloway 2013). Cu exists in three distinct forms: Cu0 (metal), Cu+ (cuprous ion), and Cu2+ (cupric ion), with cupric ion being the most poisonous and appearing element in the environment. Cu is primarily produced by extractive industries, metallurgy, chemical production, steel industries, printing circuits, electroplating industries, paints, and fertilizers (Zoroddu et al. 2019). Cu may be introduced into drinkable water as a result of the passage of water via rivers. A certain amount of copper is required by the body to maintain health, too much is hazardous. Eating or drinking an excessive amount of Cu may result in vomiting, diarrhea, abdominal cramps, nausea, liver problems, and renal illness, among other symptoms (Ko et al. 2010). It also has several negative consequences, including hair loss, anemia, renal damage, and headache. Cu builds up in the liver, brain, and pancreas, eventually resulting in death. As a result, sufficient treatment technology is required to extract the copper from the wastewater (Angelova et al. 2011).
Zinc
Zn has an important role in regulating biochemical pathways and physiological functions in living tissues. This metal is used to protect and decorate other metals by acting as a protective and ornamental layer between them. With steel, for example, the addition of Zn prevents corrosion from occurring (Rudkovskaya and Mikhailenko 2001). Steel production, mining, and coal combustion are just a few of the industrial operations that make use of this compound (Ochoa Gonzalez and Weiss 2015). Although it is necessary for humans in tiny amounts, it causes various health problems such as discomfort, vomiting, skin irritation, fever, vomiting, and anemia in some people (Plum et al. 2010). The electroplating industry, paper and pulp industries, steel manufacturing industries, and brass metal works are some of the industrial sources that produce Zn metal. The presence of Zn in quantities more than 5.0 ppm might result in a sour, astringent taste and turbulence in alkaline water. The Zn needs of humans vary because adults' Zn requirements range from 2 to 3 g. The urinary tract and the prostate have the greatest quantities of the substance. The above impacts, with the requirements for successful Zn removal from wastewater, form the basis of this research (Nriagu 2007).
Chromium
Cr is the most abundant seventh element on the planet. It is mostly produced in the leather, tanning, electroplating, and textile sectors. These businesses produce a waste product that contains hexavalent Cr(VI) and trivalent Cr(III) in varying concentrations. However, for plants, animals, and creatures, Cr(VI) is more hazardous than Cr(III). Moreover, Cr(VI) is the most prevalent element in the chromate salt manufacturing business. While Cr(III) is beneficial in lipid burning and plays a crucial role in sugar metabolism (Mishra and Bharagava 2016). Metals in these two forms are used in a variety of sectors, such as steel manufacture and chrome plating; wood conservation; the glass business; pigment fabrication; the plating and electroplating industries; and the leather tanning industry. Cr metal may also be used as cleaning chemicals, titrating agents, and additives in a variety of processes, such as mold creation and magnetic tape fabrication (Jacobs and Testa 2005). Skin irritation, liver and kidney damage, lung congestion, vomiting, and the formation of ulcers are all caused by this substance. A consequence of these effects is that Cr must be removed from wastewater before it can be released into the environment, or the Cr metal must be changed into less harmful forms before discharge into the environment (Achmad and Auerkari 2017).
Cobalt
Co may be found in minute levels in most rocks, soil, water, plants, and animals, and it is present in naturally occurring concentrations in most of these elements. It is also discovered in meteorites, which is unusual. Elemental Co is a strong, silvery-gray metal with a bluish tinge (Kosiorek and Wyszkowski 2019). Co, on the other hand, is often found in the environment in combination with other elements such as oxygen, sulfur, and As, among others. It is possible to find trace concentrations of these chemical compounds in rocks and soil as well as in plants and animals. Co may even be found in water in dissolved or ionic form but in very tiny concentrations most of the time (Banerjee and Bhattacharya 2021). A positive or negative electric charge may be found in any or both of the following: ions (atoms, groups of atoms, or molecules). Vitamin B12, also known as cyanocobalamin, is a Co molecule that is crucial in biochemistry. Vitamin B12 is required for the proper functioning of both animals and humans (Randaccio et al. 2010). When it comes to surface and groundwater within the USA, the content of Co is generally low, ranging from 1 to 10 ppb in water in crowded regions; however, concentrations can be hundreds or thousands of times higher in areas that are abundant in Co-containing mineral deposits or areas near mining as well as smelting operations. Co concentrations in most drinking water remain less than 1–2 ppb (Robertson 1968). Co is harmful to humans, as well as to terrestrial and marine animals and plants, when present in high amounts. A federal Canadian water quality standard for the preservation of freshwater or marine life against the harmful effects of Co does not exist at this time (Kosiorek and Wyszkowski 2019).
Iron
Under normal circumstances, Fe may be dissolved in water. Several Fe compounds have this feature in common. Fe pentacarbonyl, Fe hydroxide, Fe carbide, and Fe oxide are all insoluble in water in their natural forms (Kamble et al. 2013). At lower pH levels, certain Fe compounds become more water-soluble. Other Fe combinations may be more soluble in water than the ones above. When oxidized to Fe2+ under specific circumstances, they become water-soluble, but only in very acidic solutions. In both its binary and tertiary forms, Fe is an essential nutrient for most living things. However, Fe is more often found in this water-insoluble form. Water creatures in the uppermost layers of the ocean are often limited by Fe. Tertiary Fe hydroxides form when chelation ligands are missing. The World Health Organization (WHO) sets the upper limit for iron in drinking water as 0.3 mg/L (Akter et al. 2016). On the other hand, municipal and industrial waste that is dumped into water bodies leads to higher levels of iron concentrations. Drinking water with such a high quantity of iron may lead to several health problems. Too much iron in the water can cause a range of issues, such as a bad odor, an awful taste, a reddish tint to the water, and stains on clothes and plumbing fixtures. Because little is known about the dangers of waterborne iron, this is not considered to be a threat to aquatic life (Khatri et al. 2017).
Sodium
The oceans, rivers the lakes are rich in Na naturally. But the levels are significantly lower, because of the geological conditions and the poisoning of the wastewater. Na compounds are used in a wide variety of industrial processes and may end up in an industrial runoff. Water softeners used in the home may produce amounts of more than 300 mg/L (Priyadarshi 2005). The input of Na via leaching of halite via sedimentary or evaporite deposits into fluvial waters is a significantly more major source of Na in river waters. This source contributes to the Na content of fluvial waters by providing more than 40% of the Na. The Na is also formed as a result of the weathering of silicate minerals, primarily sodic plagioclase feldspar, in the environment. This source is responsible for even more than 20% of the Na in river water, according to the Environmental Protection Agency (Harring et al. 2014). It is also found in a variety of minerals such as mica, amphibole, and pyroxene. At typical levels of ingestion from a combination of food and drinking water sources, Na is a major chemical in physiological fluids, and it is not considered dangerous at these levels. Increased salt intake from drinking water, on the other hand, may be troublesome for those who have hypertension, heart disease, or renal difficulties and must adhere to a low Na diet as a result of their conditions (Fu and Wang 2011).
Lead
Pb is a toxic metal that easily accumulates in humans because of its high toxicity. Pb is a heavy and soft mineral that may be found as sulfide, cerussite (PbCl2), and galena (PbS), among other forms. The primary source of Pb contamination in industrial effluent is Pb-acid battery wastewater, which accounts for most of the problem (Pattee and Pain 2003). Electroplating, electrical, steel, and explosive factories, among other businesses, often release Pb into the environment via wastewater discharged into rivers and lakes. It also contributes to the induction of protein and DNA synthesis, as well as cell replication. It causes sickness in the human body, such as renal and neurological system damage, mental retardation, and cancer (Kaji 2004). The availability of Pb poses a greater threat to plants and animals as well. As a result, many researchers throughout the globe are currently concentrating their efforts on the development of treatment technologies for the elimination of Pb from water (Xu et al. 2020).
Mercury
Following the Hg poisoning disaster that occurred in Japan, there has been an increase in global awareness of Hg contamination. Elements such as mercury (Hg0), mercurous ion (Hg22+), and mercuric ion (Hg2+) are all known to exist in various forms. Hg may be transported and accumulated in aquatic environments. The fact that it is readily available in the environment causes several environmental issues (Schroeder and Munthe 1998). The Minamata Convention was established in 2013 to protect human health and the environment from Hg exposure. Among the many accomplishments of this convention are the regulation of Hg-containing items and the establishment of more stringent emission limits. Several Hg compounds, such as methyl mercury, have been shown to disrupt enzyme sites and impair protein synthesis (Miura et al. 1987). Considerable amounts of Hg are added in sectors such as paper and pulp, plastics, chloro-alkali, pharmaceutical, and oil refineries, among others. Besides the kidney, brain, reproductive, and respiratory systems, harm from Hg exposure is also conceivable. As a result, academics have lately focused a great deal of effort on the removal of Hg from industrial wastewater (Bernhoft 2012).
Selenium
Mineral deposits containing selenium, as well as other elements, may be found in natural deposits. However, they are also used in a variety of other items, with the most common application being electrical and photocopier components (Kieliszek and Bano 2022). Mining discharges, natural deposits, refinery discharges, and agricultural runoff all contribute to the presence of Se in drinking water. Natural Se molecules from the dry, undeveloped ground are also a source of Se pollution. Flue gas desulfurization waters, which include significant concentrations of Se, are another form of Se pollution that is well-known (Fordyce 2013) (Fig. 4).
Hazardous effects of HMs on living creators
HMs are long-lasting, hazardous even at minimal levels, and may cause severe OS in aquatic species. As a result, these pollutants have a severe ecological impact. As a result, metals cannot be degraded by microbes and hence persist in the marine environment. HMs poisoning of a river may have a disastrous impact on the aquatic ecosystem, and the variety of aquatic creatures is restricted as the contamination level increases (Khayatzadeh and Abbasi 2010). In plants, HMs can cause chlorosis, weak plant growth, and yield depression, and they may also be associated with reduced nutrient uptake, metabolic disorders, and a reduced ability to fix molecular nitrogen in leguminous plants. HMs are also known to cause toxicity and phytotoxicity in humans. The fact that seed germination was gradually delayed in the presence of increasing concentrations of Pb may be because the seeds were incubated for a longer time, which must have resulted in the neutralization of the toxic effects of lead through various mechanisms, such as leaching, chelation, metal binding, or/and accumulation by microorganisms (Bhat et al. 2019). Some of these HMs, such as As, Cr, Hg, and Pb, are not required for plant development since they do not seem to have any recognized physiological role in plants. Others, such as Co, Cu, Fe, Mn, and Zn, are vital elements for the regular development and metabolism of plants; but when their concentrations are higher than acceptable levels, these elements may readily cause poisoning. Because waste composts are mostly used to enhance soils used to produce vegetables, the use of compost to boost agricultural productivity without considering the potential negative consequences might be a concern. Because most vegetable species include edible parts of the plant, the potential of HMs transference from soil to people should be a source of worry for environmental health officials (Tchounwou et al. 2012). The microbial population that produces enzymes is shifted by HMs, which has an indirect effect on soil enzymatic activity. Toxic effects of HMs on soil biota include alteration of key microbial processes and a reduction in the population and activity of soil microorganisms. On the other hand, long-term HMs impacts might improve the tolerance of bacterial communities and fungi as arbuscular mycorrhizal (AM) fungi, which can play an essential role in the regeneration of damaged ecosystems. In polluted soils, researchers found that HMs reduced bacterial species richness and increased soil actinomycetes, or even decreased bacterial biomass and diversity (Fatima et al. 2020).
Effects of HMs on aquatic organisms
Pollutants in water can be ingested directly by aquatic species like fish, and pollutants can also be ingested indirectly through the food chain. Aquaculture provides livelihoods for hundreds of millions of people, but it also forms a significant source of plastic litter that poses a serious hazard to aquatic ecosystems (Tian et al. 2022). Fish become contaminated when chemical fertilizers with trace amounts of heavy metals are applied to the water supply. Fish have been used as a model to confirm the effects of organophosphorus and chlorinated insecticides. Fish larvae as well as juveniles grow quite quickly. Numerous environmental factors affect growth, temperature, the availability of food, and the presence of toxicants. The fish grow in length and mass when conditions are ideal, including the right temperature and an adequate supply of food. Contrarily, fish growth may be stunted in water that contains pollutants such HMs (Vajargah 2021). Growth suppression is one of the most noticeable effects of toxic metals on fish larvae. Therefore, the size and bulk of a fish can be used as indicators of its surroundings. Scientists have looked into how exposure to toxins affects DNA's health and functionality. Several biomarkers have been used as methods for detecting genotoxic pollution. DNA dimers, chromosomal abnormalities, DNA strand breaks, and counts of micronuclei are all examples of detectable biomarkers. Most commonly, erythrocytes are tested for genotoxic exposure in fish (Khayatzadeh and Abbasi 2010). Juvenile fish are especially vulnerable to the toxicity of heavy metals in water, which can drastically diminish fish stocks or even wipe them out entirely in polluted reservoirs. HMs, according to the research of numerous authors, have a detrimental effect on the development and survival of fish larvae. Vertebral abnormalities are the most common form of structural damage they cause, but they can also affect behavior (Bhat et al. 2019). A scientist conducted laboratory research on Common carp larvae in lead- or copper-contaminated water. Slower growth and developmental rate, as well as decreased survival, were observed in those exposed to heavy metals. Cu exposure prevented proper skeletal development, and Pb exposure led to scoliosis. Another researcher found that exposing common carp to HMs raised red blood cells, glucose, and cholesterol levels. Fe and Cu levels rose. During persistent exposure to hazardous heavy metals, vitamin C activity is reduced, indicating the existence of reactive oxygen species. They suggested that hazardous HMs in marine ecosystems affect common carp hematological parameters. Although they live in water, fish as well as other seafood always include certain HMs. According to the element, there are different amounts of HMs in fish that are naturally present and those that are present because of human activities. According to this ratio, fish only have a normal concentration of HMs in the open seas, which have not yet been impacted by pollution brought on by human activities (Garai et al. 2021). Estuaries, rivers, and particularly locations adjacent to industrial operations have substantially greater concentrations of HMs than is typical in polluted areas where water does not interchange with the world's seas as frequently. HMs accumulation is slow, and as a result, enormous concentrations of these metals develop in large bodies of water. Some fish species, particularly predatory species, which typically live longer, have higher levels of HMs accumulation in their various organs. A time-dependent accumulation of heavy metals poisons large creatures like goldfish or sea urchins, Pacific halibut, tuna, sharks, marlin, swordfish, and other predatory species. They may live to be 25 or older (Tchounwou et al. 2012). Skeletal muscle rarely contains excessive levels of HMs. When this occurs, it's a sign of severe environmental pollution caused by items like Cd and Hg. The liver, kidney, and bone are primarily responsible for toxic substance storage and removal in fish. These organs are rarely used for human food in Europe and the USA, with the canned cod liver being the sole exception. This is because meat and muscle tissue are what are mostly consumed (Vajargah 2021). Pb reduces thyroid hormone synthesis via effects on Fe metabolism, and Cd has been linked to reduced thyroid hormone levels, inhibition of estrogen receptors, and disruption of growth hormone production in fish. Fish may experience oxidative stress and cell membrane oxidation damage due to the prooxidative characteristics of metal ions. Similarly genotoxic to fish are the metals Cd, Cu, Hg, and Pb. Since time immemorial, the presence of HMs and other toxic substances in the water supply has posed a significant risk to both the health of humans and marine life (Tchounwou et al. 2012). After first being consumed by water microorganisms, plants, and fish, these contaminants frequently make their way into human bodies through the consumption of fisheries foods and drinking water. As a result of the deposition of various metals on the livers and kidneys of animals, particularly in limited rivers, several recent studies underscore the necessity for close monitoring of the scene (Bhat et al. 2019).
Proposed methods for the removal of HMs
Remediation using chemical and biological agents
The chemical-biological integrated treatment technique is seen as a very cost-effective and environmentally friendly solution for treating wastewater that contains HMs. Several researchers around the world have found that implementing this integrated treatment is more effective at removing HMs than doing so chemically or biologically alone. Both therapies have advantages and disadvantages when used just as an implication. Because of its ease of use and rapid turnaround time, chemical remediation has become one of the most extensively utilized remediation methods in the world (Fu and Wang 2011). However, this approach has been severely hampered by the generation of hazardous by-products and insoluble metal precipitates. Biological therapy, on the other hand, is seen as more environmentally friendly and cost-effective. Long acclimation times and variations in the biodegradability of the isolate are some of its drawbacks. With the right knowledge of each method's process, however, these limits may be eliminated (Liu et al. 2018). Many studies have found this form of an integrated system to be successful and cost-effective, which serves as a polishing step for the biological treatment and then the chemical treatment. Cr(VI) removal from textile wastewater was found to be 99.3% and 98.4% effective, respectively, using a combination of chemical precipitation and bioremediation in research by a scientist (Yadav et al. 2020). Almost 77% of the chemical oxygen demand (COD) and 81% of the turbidity were also reduced by using this method. Comparable research utilizing Fusarium chlamydosporium, a chemical precipitating and biological system, reduced turbidity by 64.69% COD by 71.80% and total chromium by 62.33%. The effectiveness of this strategy will be greatly aided by the prudent and environmentally friendly use of on-toxic chemicals (Saxena et al. 2020).
Remediation based on phytobiology
To remove HMs from soil and water, phytobial remediation is an effective and environmentally friendly option. Phytobial remediation uses plants to absorb HMs and microbes to break down those metals, as stated in the literature. As opposed to other intrusive methods, phytobiological treatment is considered to be the most environmentally friendly and cost-effective (Selvi et al. 2019). As a bonus, it may be used in large regions of polluted groundwater and soil, and sediment. As a result, its in situ treatment option has been demonstrated to reduce HMs concentrations and help preserve the topsoil. Aside from the fact that this method has many advantages, it is only suitable for shallow aquifers and soils due to the plant root length restriction, potential fear of HMs being transferred to the food chain, long duration, regular monitoring (due to litterfall), and no safe proper disposal method. According to researchers, there are a few ways to address these challenges, including employing deep-rooted plants, engineering transgenic plants that divert herbivores, and developing adequate assessment methodologies (Gajić et al. 2022). Microbes that are free to move about and interact with their environment aid in phytoremediation via mobilization, immobilization, and volatilization. Volatilization, redox alteration, leaching, and chelation are some of the processes that cause metals to get mobilized in the environment. As removal is accomplished with the employment of bacteria such as Sulfurospirillum barnesii, Geobacter, and Bacillus selenatarsenatis (Rezania et al. 2016). Aspergillus niger and Sporosarcina ginsengisoli were utilized in an immobilization approach to extract HMs from the soil. A huge number of bacteria, fungi, and algae were used in a biotransformation process to remove HMs utilizing biomethylation (Selvi et al. 2019).
Phytoremediation using electrokinetics
Remediation using electrokinetics (EK) is both more successful and more cost-efficient than the prior integrated methodologies outlined. EK treatment and its compatibility with phytoremediation were the driving forces for this combination. However, in situ phytoremediation is restricted by factors like conditions, metallic accessibility, and shallow depths when applied as an individual operation (Yuan and Weng 2006). The restoration yield and the processing speed must also be improved. It is possible, however, to combine phytoremediation with other tactics like bioaugmentation, and remediation using electrokinetic and permeable reactive barriers to increase the effectiveness. Zn, Pb, Cu, Cd, and As HMs remediation laboratory research using EK and phytoremediation technique have shown a promising vision. Phytoremediation also relies heavily on electrokinetics. Organic and inorganic molecules may be separated by passing a direct current between electrodes positioned vertically in the soil. Plant absorption processes dictated which phytoremediation procedures were used, such as phytoextraction, phytoevaporation, rhizodegradation, and rhizofiltration. Researchers have used electrokinetic coupled phytoremediation to remove hazardous As from water using the species Lemna minor (Cameselle et al. 2013). For this test, sodium arsenate was used at an amount of 150 g L1 to create fake As water. After the trial, their early data revealed a 90% clearance rate. To promote phytoremediation and avoid mobile metals from leaking to the ground surface, EK remediation research used several electrode configurations to deploy numerous electrode configurations to enhance Pb removal from soil (Kuppusamy et al. 2016).
Low-cost methods to overcome hazardous HMs from water
Aquatic and terrestrial biomass
Bioremediation has also been investigated for a variety of trees, plants, and other lands- and water-based materials abundant in poor nations. For example, the potential capacity of Moringa oleifera (MO) to remove HMs has drawn much interest. The water and wastewater treatment capabilities of the tropical drought-tolerant tree MO have been investigated. MO is very effective in removing from water and wastewater a broad range of HMs, including Cd(II), Fe(II), Cr(III), Zn(II), and Cu(II) (Dixit et al. 2015). Additionally, researchers have shown that MO does not have a major impact on the water's characteristics (e.g., pH, ionic strength) when used in conjunction with HMs removal. Adsorption, charge neutralization, complexation, and interparticle bridging are some of the removal processes for MO that have been suggested. Sawdust is another substance that has been extensively studied for its adsorption properties. For HMs removal, the sawdust from various trees is beneficial in several experiments. Cr(VI) removal (> 80%) was obtained by maple sawdust, whereas beech sawdust efficiently removed Cu(II), Zn(II), and Mn(II) respectively. HMs may be efficiently removed from a wide range of water sources using aquatic biomass, including seaweed and algae. For example, 2 different freshwater microalgae, S. Condensata and R. Hieroglyphicum, have been shown to successfully remove Cr(III) from industrial effluent, with maximal adsorption capacities of 14.8 and 12.5 mg g−1, respectively (Ojuederie and Babalola 2017). In another work, an artificially grown marine alga, U. pinnatifida, displayed efficient removal of Ni(II) and Cu(II), with maximal adsorption capacities of 29.9 and 78.9 mg g−1, correspondingly. The removal of Cd(II), Zn(II), and other HMs from water utilizing brown (A. nodosum), red, and green algae in a variety of combinations and systems was recently studied by scientists. Every time, the removal of HMs (> 90%) was successful, no matter how they were combined (Joseph et al. 2019). According to research on the removal of Cd(II) using brown, red, and green seaweed, there was a broad variety of adsorption capabilities. Adsorption capabilities varied from 17.9 mg g−1 for brown seaweeds to 82.9 mg g−1 for green and red seaweeds. Shells from a variety of aquatic animals have been found in HMs from water. Cu(II) and Pb(II) were efficiently removed from water by shells from the mollusk Anadara inaequivalvis (A. inaequivalvis), which are commonly found in the Adriatic, Aegean, and Black Seas (Freitas et al. 2008).
Agricultural waste
Scientists both in the advanced and developing worlds have conducted extensive studies into the use of agricultural residues to remove HMs from the environment. The removal of HMs from water in the setting of a developing nation is often made possible by the use of agricultural waste, which is a readily available supply of efficient adsorbents that can be used in water treatment procedures (Upendra 2006). Dairy manure compost is a unique substance that has been demonstrated to be very successful in removing HMs from the environment, with maximum adsorption capacities of 15.5, 27.2, and 95.3 mg g−1 for Zn, Cu, and Pb, among other HMs, according to research (Saxena et al. 2020). Rice residual waste materials are a common kind of agricultural trash that is generated in enormous quantities, especially in developing countries, and are a common source of agricultural waste. Rice bran, rice straw, and rice husk are all examples of this sort of trash. It has been shown that these waste products are efficient in removing HMs from aqueous solutions. Using rice straw and rice bran, for example, it has been shown that Cu(II) can be removed from the solution with maximal adsorption capacities of 18.4 and 21.0 mg g−1, respectively (Chuah et al. 2005). Several other studies have also shown that rice husk has the potential to remove HMs from water sources. It has also been shown that the waste from nuts may be used to eliminate HMs from various water sources. Cashew nut shells have been studied for their potential to eliminate HMs from water. Researchers found that cashew nut shells had a maximum adsorption capacity of 20 mg g−1 when measuring the removal of Cu(II). Cashew nut shells were used in another investigation to remove nickel(II) from water, and the researchers found that they had a maximum adsorption capacity of 18.9 mg g−1. Due to the large surface area of cashew nut shells, adsorption of these HMs may occur on a large number of active adsorption sites (Upendra 2006). It has been shown that fruit wastes are good in removing HMs from water. The highest adsorption capabilities of lemon peel were found to be 27.9 mg g−1 Zn(II), 37.9 mg g−1 Pb(II), and 71.0 mg g−1 Cu respectively. It has also been shown that grape stalk waste has the potential to remove HMs, with maximal adsorption capacities of 10.1 and 10.6 mg g−1 obtained for Cu(II) and Ni(II). Vegetable waste from other sources has been found to remove HMs from water. Heavier metals may be removed with the use of mushroom remnants (Sabir et al. 2021). Cu(II), Zn(II), and Hg(II) removal efficiencies varied from 39.7 to 81.7% based on an evaluation of four distinct kinds of mushroom residues. Another research evaluated the removal of Cd(II) and Pb(II) using three distinct mushrooms and obtained maximal adsorption capacities of 35.0 and 33.8 mg g−1, respectively. Corncob also effectively removed Pb(II), with an adsorption capacity of 16.2 mg g−1 at its highest. Sodium hydroxide treatment of corncob boosted its Pb(II) adsorption capacity by more than fourfold (43.4 mg g−1) (Al-Qodah et al. 2017).
Conclusion
This review aimed to present the importance of reservoirs and temperature effect as quantitative and qualitative water reserves in watersheds; analyze the fundamental characteristics of these artificial ecosystems; and propose new technologies and approaches for reservoir location, construction, and operation based on the scientific knowledge accumulated. To meet the demand for fish from a growing global population, aquaculture production must be increased due to the stagnation of capture fisheries. However, aquaculture has generated a wide range of environmental challenges, and further environmental effects are likely if aquaculture production continues. Climate change is a further threat to global fish production. Higher aquaculture production is needed that is environmentally sustainable and can adapt to climate change. There is a major shortage of access to safe drinking water in developing countries due to water scarcity and polluted water. HMs poisoning in water sources is becoming a major issue, notwithstanding the existence of other kinds of water pollution. HMs present in compost have the potential to alter the physical, chemical, and biological aspects of the soil and water. Because of the absorption of these metals by plants from the soil, crop yield is reduced because of the inhibition of physiological metabolism. It is both human health and the environment that are concerned about HMs being taken up by fish and accumulate in human tissues, as well as bio-magnifications as they go up the food chain. Aquatic animals are harmed by HMs found in agricultural runoff that enters the aquatic environment. When compost is used in agriculture, it should not include pathogens or HMs since they are detrimental to the crop. It is also important to conduct bench-scale testing of these materials with real, local water sources such as industrial effluent, river and lake sources, and home wastewater. Social, economic, and ecological challenges must also be identified and addressed to facilitate the proposed adaptation strategies. Empirical research is needed to understand interlinked processes of increasing aquaculture productivity, environmental sustainability, and climate change adaptability.
Availability of data and material
All data are included.
References
Achmad RT, Auerkari EI (2017) Effects of chromium on human body. Annu Res Rev Biol 17:1–8
Akter T, Jhohura FT, Akter F, Chowdhury TR, Mistry SK, Dey D, Barua MK, Islam MA, Rahman M (2016) Water quality index for measuring drinking water quality in rural bangladesh: a cross-sectional study. J Health Popul Nutr 35(1):1–12
Alam MG, Allinson G, Stagnitti F, Tanaka A, Westbrooke M (2002) Arsenic contamination in bangladesh groundwater: a major environmental and social disaster. Int J Environ Health Res 12(3):235–253
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ (2021) Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics 9(3):42
Ali H, Khan E, Ilahi I (2019a) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem 2019:1–4
Ali R, Kuriqi A, Abubaker S, Kisi O (2019b) Hydrologic alteration at the upper and middle part of the Yangtze river, China: towards sustainable water resource management under increasing water exploitation. Sustainability 11(19):5176
Ali MM, Hossain D, Al-Imran M, Khan S, Begum M, Osman MH (2021) environmental pollution with heavy metals: a public health concern. In: Nazal MK, Zhao H (eds) Heavy metals—their environmental impacts and mitigation. IntechOpen, London
Alloway BJ (2013) Heavy metals and metalloids as micronutrients for plants and animals. In: Alloway BJ (ed) Heavy metals in soils. Springer, Dordrecht, pp 195–209
Al-Qodah Z, Yahya MA, Al-Shannag M (2017) On the performance of bioadsorption processes for heavy metal ions removal by low-cost agricultural and natural by-products bioadsorbent: a review. Desalin Water Treat 85:339–357
Angelova M, Asenova S, Nedkova V, Koleva-Kolarova R (2011) Copper in the human organism. Trakia J Sci 9(1):88–98
Ashmore P, Sauks E (2006) Prediction of discharge from water surface width in a braided river with implications for at-a-station hydraulic geometry. Water Resour Res 42(3):1–11
Bakken TH, King T, Alfredsen K (2016) Simulation of river water temperatures during various hydro-peaking regimes. J Appl Water Eng Res 4(1):31–43
Bakker ES, Hilt S (2016) Impact of water-level fluctuations on cyanobacterial blooms: options for management. Aquat Ecol 50:485–498
Balogh SJ, Engstrom DR, Almendinger JE, McDermott C, Hu J, Nollet YH, Meyer ML, Kent Johnson D (2009) A sediment record of trace metal loadings in the Upper Mississippi River. J Paleolimnol 41(4):623–639. https://doi.org/10.1007/s10933-008-9295-2
Banerjee P, Bhattacharya P (2021) Investigating cobalt in soil–plant–animal–human system: dynamics, impact and management. J Soil Sci Plant Nutr 21(3):2339–2354
Beechie T, Buhle E, Ruckelshaus M, Fullerton A, Holsinger L (2006) Hydrologic regime and the conservation of salmon life history diversity. Biol Conserv 130(4):560–572
Bernhoft RA (2012) Mercury toxicity and treatment: a review of the literature. J Environ Public Health 2012:1–11
Bhat SA, Hassan T, Majid S (2019) Heavy metal toxicity and their harmful effects on living organisms–a review. Int J Med Sci Diagn Res 3(1):106–122
Biney CA, Amuzu AT, Calamari D, Kaba N, Mbome IL, Naeve H, Ochumba PB, Osibanjo O, Radegonde V, Saad MA (1994) Review of heavy metals in the african aquatic environment. Ecotoxicol Environ Saf 28(2):134–159
Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S (2013) Climate change and the past, present, and future of biotic interactions. Science 341(6145):499–504
Boulange J, Hanasaki N, Yamazaki D, Pokhrel Y (2021) Role of dams in reducing global flood exposure under climate change. Nat Commun 12(1):417
Bradl HB (2005) Sources and origins of heavy metals. In: Saleh TA, Gupta VK (eds) Interface science and technology, vol 6. Elsevier, Amsterdam, pp 1–27
Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson A, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10(8):701–709
Caissie D (2006) The thermal regime of rivers: a review. Freshw Biol 51(8):1389–1406
Cameselle C, Chirakkara RA, Reddy KR (2013) Electrokinetic-enhanced phytoremediation of soils: status and opportunities. Chemosphere 93(4):626–636
Cardinale BJ, Ives AR, Inchausti P (2004) Effects of species diversity on the primary productivity of ecosystems: extending our spatial and temporal scales of inference. Oikos 104(3):437–450
Carter DE, Aposhian HV, Gandolfi AJ (2003) The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: a toxicochemical review. Toxicol Appl Pharmacol 193(3):309–334
Chuah TG, Jumasiah A, Azni I, Katayon S, Choong ST (2005) Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: an overview. Desalination 175(3):305–316
Collischonn W, Allasia D, Da Silva BC, Tucci CE (2007) The MGB-IPH model for large-scale rainfall—runoff modelling. Hydrol Sci J 52(5):878–895
Cooper AR, Infante DM, Daniel WM, Wehrly KE, Wang L, Brenden TO (2017) Assessment of dam effects on streams and fish assemblages of the conterminous USA. Sci Total Environ 586:879–889
Dallas HF, Rivers-Moore NA (2012) Critical thermal maxima of aquatic macroinvertebrates: towards identifying bioindicators of thermal alteration. Hydrobiologia 679:61–76
Datry T (2012) Benthic and hyporheic invertebrate assemblages along a flow intermittence gradient: effects of duration of dry events. Freshw Biol 57(3):563–574
Dimitriadis P, Koutsoyiannis D, Iliopoulou T, Papanicolaou P (2021) A global-scale investigation of stochastic similarities in marginal distribution and dependence structure of key hydrological-cycle processes. Hydrology 8(2):59
Dixit R, Malaviya D, Pandiyan K, Singh UB, Sahu A, Shukla R, Singh BP, Rai JP, Sharma PK, Lade H, Paul D (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7(2):2189–2212
Duffus JH (2002) ‘Heavy metals’ a meaningless term? (IUPAC technical report). Pure Appl Chem 74(5):793–807
Fatima S, Muzammal M, Rehman A, Rustam SA, Shehzadi Z, Mehmood A, Waqar M (2020) Water pollution on heavy metals and its effects on fishes. Int J Fish Aquat Stud 8:6–14
Flanagan NE, Richardson CJ, Ho M (2015) Connecting differential responses of native and invasive riparian plants to climate change and environmental alteration. Ecol Appl 25(3):753–767
Fordyce FM (2013) Selenium deficiency and toxicity in the environment. In: Selinus O (ed) Essentials of medical geology. Springer, Berlin, pp 375–416
Freitas OM, Martins RJ, Delerue-Matos CM, Boaventura RA (2008) Removal of Cd (II), Zn (II) and Pb (II) from aqueous solutions by brown marine macro algae: kinetic modelling. J Hazard Mater 153(1–2):493–501
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418
Fu B-J, Wu B-F, Lue Y-H, Xu Z-H, Cao J-H, Niu D, Yang G-S, Zhou Y-M (2010) Three gorges project: efforts and challenges for the environment. Prog Phys Geogr 34(6):741–754
Gajić G, Mitrović M, Pavlović P (2022) Phytobial remediation by bacteria and fungi. In: Pandey V (ed) Assisted phytoremediation. Elsevier, Amsterdam, pp 285–344
Garai P, Banerjee P, Mondal P, Saha NC (2021) Effect of heavy metals on fishes: toxicity and bioaccumulation. J Clin Toxicol 11(August):1
Gebrekiros ST (2016) Factors affecting stream fish community composition and habitat suitability. J Aquacult Mar Biol 4(2):00076
Ghermandi A, Vandenberghe V, Benedetti L, Bauwens W, Vanrolleghem PA (2009) Model-based assessment of shading effect by riparian vegetation on river water quality. Ecol Eng 35(1):92–104
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J Energy Environ 6(4):18
Gorczyc E, Krzemień K, Liro M, Sobucki M (2017) Changes of mountain river channels and their environmental effects. In: Radecki-Pawlik A, Pagliara S, Hradecky J (eds) Open channel hydraulics, river hydraulic structures and fluvial geomorphology. CRC Press, Boca Raton, pp 303–321
Graeme KA, Pollack CV Jr (1998) Heavy metal toxicity, part I: arsenic and mercury. J Emerg Med 16(1):45–56
Gu L, Pallardy SG, Hosman KP, Sun Y (2016) Impacts of precipitation variability on plant species and community water stress in a temperate deciduous forest in the central US. Agric for Meteorol 217:120–136
Guo H, Hu Q, Zhang Q, Feng S (2012) Effects of the Three Gorges Dam on Yangtze river flow and river interaction with Poyang Lake, China: 2003–2008. J Hydrol 416:19–27
Gupta DK, Sarita Tiwari BHN, Razafindrabe SC (2017) Arsenic contamination from historical aspects to the present. In: Gupta DK, Chatterjee S (eds) Arsenic contamination in the environment. Springer, Cham, pp 1–12
Hao J, Wang S (2009) Control techniques for particles. In: Yi Q (ed) Point sources of pollution: local effects and control. Eolss Publishers Company Limited, Oxford, pp 194–222
Harring TR, Deal NS, Kuo DC (2014) Disorders of sodium and water balance. Emerg Med Clin 32(2):379–401
Huang G, Izumiya T (2003) Maintaining natural water temperature regime for protecting aquatic life. Environ Syst Res 31:159–168
Huang X-R, Gao L-Y, Yang P-P, Xi Y-Y (2018) Cumulative impact of dam constructions on streamflow and sediment regime in lower reaches of the Jinsha River, China. J Mt Sci 15(12):2752–2765
Jacobs JA, Testa SM (2005) Overview of chromium(VI) in the environment: background and history. In: Guertin J, Avakian C, Jacobs J (eds) Chromium(VI) handbook. CRC Press, Boca Raton, pp 1–21
Jadia CD, Fulekar MH (2009) Phytoremediation of heavy metals: recent techniques. Afr J Biotechnol 8(6):921–928
Jeppesen E, Brucet S, Naselli-Flores L, Papastergiadou E, Stefanidis K, Noges T, Noges P, Attayde JL, Zohary T, Coppens J, Bucak T (2015) Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 750:201–227
Johnstone JF, Allen CD, Franklin JF, Frelich LE, Harvey BJ, Higuera PE, Mack MC, Meentemeyer RK, Metz MR, Perry GL, Schoennagel T (2016) Changing disturbance regimes, ecological memory, and forest resilience. Front Ecol Environ 14(7):369–378
Joseph L, Jun B-M, Flora JRV, Park CM, Yoon Y (2019) Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere 229:142–159
Kabel K, Moros M, Porsche C, Neumann T, Adolphi F, Andersen TJ, Siegel H, Gerth M, Leipe T, Jansen E, Sinninghe Damsté JS (2012) Impact of climate change on the Baltic Sea ecosystem over the past 1,000 years. Nat Clim Change 2(12):871–874
Kahlon SK, Gaurav Sharma JM, Julka AK, Sharma S, Stadler FJ (2018) Impact of heavy metals and nanoparticles on aquatic biota. Environ Chem Lett 16(3):919–946
Kaji T (2004) Cell biology of heavy metal toxicity in vascular tissue. Yakugaku Zasshi J Pharm Soc Jpn 124(3):113–120
Kamble R, Thakare MG, Ingle AB (2013) Iron in the environment. Indian J Environ Prot 33:881–888
Kar D, Sur P, Mandai SK, Saha T, Kole RK (2008) Assessment of heavy metal pollution in surface water. Int J Environ Sci Technol 5(1):119–124
Khatri N, Tyagi S, Rawtani D (2017) Recent strategies for the removal of iron from water: a review. J Water Process Eng 19:291–304
Khayatzadeh J, Abbasi E (2010) The effects of heavy metals on aquatic animals. In: The 1st international applied geological congress, Department of Geology, Islamic Azad University–Mashad Branch, Iran 1(April), pp 26–28
Khurram D, Bao Y, Tang Q, He X, Li J, de Nambajimana J, Nsabimana G (2023) Sedimentary geochemistry mediated by a specific hydrological regime in the water level fluctuation zone of the Three Gorges Reservoir, China. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-25086-y
Kieliszek M, Bano I (2022) Selenium as an important factor in various disease states: a review. EXCLI J 21:948–966
Ko S, Olaiya CO, Oyewole EO (2010) The importance of mineral elements for humans, domestic animals and plants-a review. African J Food Sci 4(5):200–222
Kosiorek M, Wyszkowski M (2019) Effect of cobalt on the environment and living organisms—a review. Appl Ecol Environ Res 17(5):11419–11449
Kuppusamy S, Palanisami T, Megharaj M, Venkateswarlu K, Naidu R (2016) In-situ remediation approaches for the management of contaminated sites: a comprehensive overview. In: de Voogt P (ed) Reviews of environmental contamination and toxicology, vol 236. Springer, Cham, pp 1–115
Kurylyk BL, Irvine DJ, Bense VF (2019) Theory, tools, and multidisciplinary applications for tracing groundwater fluxes from temperature profiles. Wiley Interdiscip Rev Water 6(1):e1329
Lajoie F, Assani AA, Roy AG, Mesfioui M (2007) Impacts of dams on monthly flow characteristics. The influence of watershed size and seasons. J Hydrol 334(3–4):423–439
Lal R (2012) Climate change and soil degradation mitigation by sustainable management of soils and other natural resources. Agric Res 1:199–212
Lehner B, Liermann CR, Revenga C, Vörösmarty C, Fekete B, Crouzet P, Döll P, Endejan M, Frenken K, Magome J, Nilsson C (2011) High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front Ecol Environ 9(9):494–502
Leibowitz SG (2003) Isolated wetlands and their functions: an ecological perspective. Wetlands 23(3):517–531
Leira M, Cantonati M (2008) Effects of water-level fluctuations on lakes: an annotated bibliography. In: Wantzen KM, Rothhaupt K-O, Mörtl M, Cantonati M, Tóth LG, Fischer P (eds) Ecological effects of water-level fluctuations in lakes. Springer, Dordrecht, pp 171–184
Li Q, Lai G, Devlin AT (2021) A review on the driving forces of water decline and its impacts on the environment in Poyang Lake, China. J Water Clim Change 12(5):1370–1391
Lin X, Lu K, Hardison AK, Liu Z, Xu X, Gao D, Gong J, Gardner WS (2021) Membrane inlet mass spectrometry method (REOX/MIMS) to measure 15N-nitrate in isotope-enrichment experiments. Ecol Indic 126:107639
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219
Liu M, Zhang Y, Shi K, Zhu G, Wu Z, Liu M, Zhang Y (2019) Thermal stratification dynamics in a large and deep subtropical reservoir revealed by high-frequency buoy data. Sci Total Environ 651:614–624
Liu Y, Zhang K, Li Z, Liu Z, Wang J, Huang P (2020) A hybrid runoff generation modelling framework based on spatial combination of three runoff generation schemes for semi-humid and semi-arid watersheds. J Hydrol 590:125440
Liu S, Hou J, Suo C, Chen J, Liu X, Fu R, Wu F (2022) Molecular-level composition of dissolved organic matter in distinct trophic states in Chinese lakes: Implications for eutrophic lake management and the global carbon cycle. Water Res 217:118438
Liyana-Arachchi TP, Zhang Z, Vempati H, Hansel AK, Stevens C, Pham AT, Ehrenhauser FS, Valsaraj KT, Hung FR (2014) Green leaf volatiles on atmospheric air/water interfaces: a combined experimental and molecular simulation study. J Chem Eng Data 59(10):3025–3035
Lucas C, Hennessy K, Mills G, Bathols J (2007) Bushfire weather in southeast Australia: recent trends and projected climate change impacts
Magalhaes MF, Beja P, Schlosser IJ, Collares-Pereira MJ (2007) Effects of multi-year droughts on fish assemblages of seasonally drying Mediterranean streams. Freshw Biol 52(8):1494–1510
Magilligan FJ, Nislow KH (2005) Changes in hydrologic regime by dams. Geomorphology 71(1–2):61–78
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58(1):201–235
Manzoor M, Bhat KA, Khurshid N, Yatoo AM, Zaheen Z, Ali S, Ali MN, Amin I, Mir MU, Rashid SM, Rehman MU (2021) Bio-indicator species and their role in monitoring water pollution. In: Dar GH, Hakeem KR, Mehmood MA, Qadri H (eds) Freshwater pollution and aquatic ecosystems. Apple Academic Press, New York, pp 321–347
Masindi V, Muedi KL (2018) Environmental contamination by heavy metals. Heavy Met 10:115–132
Mayor SJ, Schneider DC, Schaefer JA, Mahoney SP (2009) Habitat selection at multiple scales. Ecoscience 16(2):238–247
McManus DP, Gray DJ, Li Y, Feng Z, Williams GM, Stewart D, Rey-Ladino J, Ross AG (2010) Schistosomiasis in the People’s Republic of China: the era of the Three Gorges Dam. Clin Microbiol Rev 23(2):442–466
Mei X, Dai Z, Darby SE, Gao S, Wang J, Jiang W (2018) Modulation of extreme flood levels by impoundment significantly offset by floodplain loss downstream of the Three Gorges Dam. Geophys Res Lett 45(7):3147–3155
Mezger G, del Tánago MG, De Stefano L (2021) Environmental flows and the mitigation of hydrological alteration downstream from dams: the Spanish case. J Hydrol 598:125732
Mishra S, Bharagava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health Part C 34(1):1–32
Miura K, Imura N, Clarkson TW (1987) Mechanism of methylmercury cytotoxicity. CRC Crit Rev Toxicol 18(3):161–188
Müller P, Bonthuis DJ, Miller R, Schneck E (2021) Ionic surfactants at air/water and oil/water interfaces: a comparison based on molecular dynamics simulations. J Phys Chem B 125(1):406–415
Nathan R, Jordan P, Morden R (2005) Assessing the impact of farm dams on streamflows, Part I: development of simulation tools. Aust J Water Resour 9(1):1–12
New T, Xie Z (2008) Impacts of large dams on riparian vegetation: applying global experience to the case of China’s Three Gorges Dam. Biodivers Conserv 17:3149–3163
Nikora VI, Rowiński P, Suchodolov A, Krasuski D (1994) Structure of river turbulence behind warm-water discharge. J Hydraul Eng 120(2):191–208
Nriagu JO (1990) Global metal pollution: Poisoning the biosphere? Environ Sci Policy Sustain Dev 32(7):7–33
Nriagu J (2007) Zinc toxicity in humans. School of Public Health, University of Michigan, Ann Arbor, pp 1–7
Ochoa Gonzalez R, Weiss D (2015) Zinc isotope variability in three coal-fired power plants: a predictive model for determining isotopic fractionation during combustion. Environ Sci Technol 49(20):12560–12567
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14(12):1504
Omofunmi O, Kolo J, Oladipo A, Diabana P, Ojo A (2017) A review on effects and control of seepage through earth-fill dam. Curr J Appl Sci Technol 22(5):1–11
Pattee OH, Pain DJ (2003) Lead in the environment. Handb Ecotoxicol 2:373–399
Piechota G, Igliński B, Buczkowski R (2013) Development of measurement techniques for determination main and hazardous components in biogas utilised for energy purposes. Energy Convers Manag 68:219–226
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7(4):1342–1365
Poff NL, Olden JD, Merritt DM, Pepin DM (2007) Homogenization of regional river dynamics by dams and global biodiversity implications. Proc Natl Acad Sci 104(14):5732–5737
Pringle CM (2001) Hydrologic connectivity and the management of biological reserves: a global perspective. Ecol Appl 11(4):981–998
Priyadarshi N (2005) Sodium in natural waters. Water Encycl 4:551–553
Pujari M, Kapoor D (2021) Heavy metals in the ecosystem: sources and their effects. In: Kumar V, Sharma A, Cerdà A (eds) Heavy metals in the environment. Elsevier, Amsterdam, pp 1–7
Quan Q, Liang W, Yan D, Lei J (2022) Influences of joint action of natural and social factors on atmospheric process of hydrological cycle in Inner Mongolia, China. Urban Clim 41:101043
Randaccio L, Geremia S, Demitri N, Wuerges J (2010) Vitamin B12: unique metalorganic compounds and the most complex vitamins. Molecules 15(5):3228–3259
Ranjan A (2018) China’s water problems. Asian Affairs 49(4):645–658
Rezania S, Taib SM, Din MFM, Dahalan FA, Kamyab H (2016) Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater 318:587–599
Rilov G, Figueira W, Lyman S, Crowder L (2007) Complex habitats may not always benefit prey: linking visual field with reef fish behavior and distribution. Mar Ecol Prog Ser 329:225–238
Robertson DE (1968) Role of Contamination in trace element analysis of sea water. Anal Chem 40(7):1067–1072
Rogosch JS, Tonkin JD, Lytle DA, Merritt DM, Reynolds LV, Olden JD (2019) Increasing drought favors nonnative fishes in a dryland river: evidence from a multispecies demographic model. Ecosphere 10(4):e02681
Rosenberry DO, Morin RH (2004) Use of an electromagnetic seepage meter to investigate temporal variability in lake seepage. Groundwater 42(1):68–77
Rudkovskaya NV, Mikhailenko NY (2001) Decorative zinc-containing crystalline glazes for ornamental ceramics (a review). Glass Ceram 58(11):387–390
Sabater S (2008) Alterations of the global water cycle and their effects on river structure, function and services. Freshw Rev 1(1):75–88
Sabir A, Altaf F, Batool R, Shafiq M, Khan RU, Jacob KI (2021) Agricultural waste absorbents for heavy metal removal. In: Inamuddin MI, Ahamed EL, Asiri AbM (eds) Green adsorbents to remove metals, dyes and boron from polluted water. Springer, Cham, pp 195–228
Sankhla MS, Kumari M, Nandan M, Kumar R, Agrawal P (2016) Heavy metals contamination in water and their hazardous effect on human health-a review. Int J Curr Microbiol Appl Sci 5(10):759–766
Saxena G, Purchase D, Bharagava RN (2020) Environmental hazards and toxicity profile of organic and inorganic pollutants of tannery wastewater and bioremediation approaches. In: Saxena G, Bharagava RN (eds) Bioremediation of industrial waste for environmental safety: volume I: industrial waste and its management. Springer, Singapore, pp 381–398
Schroeder WH, Munthe J (1998) Atmospheric mercury—an overview. Atmos Environ 32(5):809–822
Selvi A, Rajasekar A, Theerthagiri J, Ananthaselvam A, Sathishkumar K, Madhavan J, Rahman PK (2019) Integrated remediation processes toward heavy metal removal/recovery from various environments-a review. Front Environ Sci 7:66
Shen Z, Qiu J, Hong Q, Chen L (2014) Simulation of spatial and temporal distributions of non-point source pollution load in the Three Gorges Reservoir Region. Sci Environ 493:138–146
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43(3):246
Sinokrot BA, Stefan HG (1993) Stream temperature dynamics: measurements and modeling. Water Resour Res 29(7):2299–2312
Smedley PL (2003) Arsenic in groundwater—south and east Asia. In: Welch AH, Stollenwerk KG (eds) Arsenic in ground water. Springer, Berlin, pp 179–209
Soomro SE, Hu C, Boota MW, Ahmed Z, Chengshuai L, Zhenyue H, Xiang L, Soomro MH (2022) River flood susceptibility and basin maturity analyzed using a coupled approach of geo-morphometric parameters and SWAT model. Water Resour Manag 36(7):2131–2160
Soomro S, Shi X, Guo J, Hu C, Zwain HM, Liu C, Khan MZ, Niu C, Zhao C, Ahmed Z (2023) Appraisal of climate change and source of heavy metals, sediments in water of the Kunhar river watershed, Pakistan. Nat Hazards. https://doi.org/10.1007/s11069-022-05760-7
Studds CE, Marra PP (2007) Linking fluctuations in rainfall to nonbreeding season performance in a long-distance migratory bird, Setophaga ruticilla. Clim Res 35(1–2):115–122
Tang Q, Bao Y, He X, Fu B, Collins AL, Zhang X (2016) Flow regulation manipulates contemporary seasonal sedimentary dynamics in the reservoir fluctuation zone of the Three Gorges Reservoir, China. Sci Total Environ 548:410–420
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. In: Luch A (ed) Molecular, clinical and environmental toxicology. Springer, Basel, pp 133–164
Thuiller W, Midgley GF, Hughes GO, Bomhard B, Drew G, Rutherford MC, Woodward FI (2006) Endemic species and ecosystem sensitivity to climate change in Namibia. Glob Change Biol 12(5):759–776
Tian Y, Yang Z, Yu X, Jia Z, Rosso M, Dedman S, Zhu J, Xia Y, Zhang G, Yang J, Wang J (2022) Can we quantify the aquatic environmental plastic load from aquaculture? Water Res 219:118551
Upendra K (2006) Agricultural products and by-products as a low cost adsorbent for heavy metal removal from water and wastewater: a review. Sci Res Essay 1(2):33–37
Vajargah MF (2021) A review on the effects of heavy metals on aquatic animals. J Biomed Res Environ Sci 2(9):865–869
Verma R, Dwivedi P (2013) Heavy metal water pollution-a case study. Recent Res Sci Technol 5(5):98–99
Wang J, Sheng Y, Gleason CJ, Wada Y (2013) Downstream Yangtze River levels impacted by three Gorges dam. Environ Res Lett 8(4):044012
Wang J, Sheng Y, Tong TSD (2014) Monitoring decadal lake dynamics across the Yangtze Basin downstream of Three Gorges Dam. Remote Sens Environ 152:251–269
Wang Y, Rhoads BL, Wang D (2016) Assessment of the flow regime alterations in the middle reach of the Yangtze River associated with dam construction: potential ecological implications. Hydrol Process 30(21):3949–3966
Webb B, Zhang Y (1997) Spatial and seasonal variability in the components of the river heat budget. Hydrol Process 11(1):79–101
Willems P, Vrac M (2011) Statistical precipitation downscaling for small-scale hydrological impact investigations of climate change. J Hydrol 402(3–4):193–205
Winton RS, Calamita E, Wehrli B (2019) Reviews and syntheses: dams, water quality and tropical reservoir stratification. Biogeosciences 16(8):1657–1671
Wu S, Yin Y, Zhao D, Huang M, Shao X, Dai E (2010) Impact of future climate change on terrestrial ecosystems in China. Int J Climatol J R Meteorol Soc 30(6):866–873
Wu J, Long J, Liu M (2015) Evolving RBF neural networks for rainfall prediction using hybrid particle swarm optimization and genetic algorithm. Neurocomputing 148:136–142
Xu K, Milliman JD (2009) Seasonal variations of sediment discharge from the Yangtze River before and after impoundment of the Three Gorges Dam. Geomorphology 104(3–4):276–283
Xu C, Liu J, Bi Y, Ma C, Bai J, Hu Z, Zhou M (2020) Biomass derived worm-like nitrogen-doped-carbon framework for trace determination of toxic heavy metal lead (II). Anal Chim Acta 1116:16–26
Yadav M, Gupta R, Arora G, Yadav P, Srivastava A, Sharma RK (2020) Current status of heavy metal contaminants and their removal/recovery techniques. In: Ahuja S, Loganathan BG (eds) Contaminants in our water: identification and remediation methods. ACS Publications, Washington, pp 41–64
Yan H, Zhang X, Xu Q (2021a) Variation of runoff and sediment inflows to the Three Gorges Reservoir: impact of upstream cascade reservoirs. J Hydrol 603:126875
Yan Q, Jing Z, Xia Z, Zhang S (2021b) Flood control capacity of the Three Gorges Project for different frequency floods. Environ Eng Sci 38(12):1195–1205
Yang Z, Yu X, Dedman S, Rosso M, Zhu J, Yang J, Xia Y, Tian Y, Zhang G, Wang J (2022) UAV remote sensing applications in marine monitoring: Knowledge visualization and review. Sci Total Environ 838:155939
Yuan C, Weng C-H (2006) Electrokinetic enhancement removal of heavy metals from industrial wastewater sludge. Chemosphere 65(1):88–96
Zanjani MM, Soroush A, Khoshini M (2016) Two-dimensional numerical modeling of fault rupture propagation through earth dams under steady state seepage. Soil Dyn Earthq Eng 88:60–71
Zhang Q (2015) China's 2003 Environmental impact assessment law and dams: How effective is it?
Zhang Q, Lou Z (2011) The environmental changes and mitigation actions in the Three Gorges Reservoir region, China. Environ Sci Policy 14(8):1132–1138
Zhang Q, Xu C-Y, Yang T (2009) Variability of water resource in the Yellow River basin of past 50 years, China. Water Resour Manag 23:1157–1170
Zhang X, Ma F, Yin S, Wallace CD, Soltanian MR, Dai Z, Ritzi RW, Ma Z, Zhan C, Lü X (2021) Application of upscaling methods for fluid flow and mass transport in multi-scale heterogeneous media: a critical review. Appl Energy 303:117603
Zheng S, Zhong Z, Zou Q, Ding Y, Yang L, Luo X, Zheng S, Zhong Z, Zou Q, Ding Y, Yang L (2021) Study on regulation mode of small and medium floods in the Three Gorges Project. In: Zheng S, Zhong Z, Zou Q, Ding Y, Yang L, Luo X (eds) Flood Resources utilization in the Yangtze River basin. Springer, Singapore, pp 235–313
Zhou Y-B, Liang S, Chen Y, Jiang Q-W (2016) The Three Gorges Dam: Does it accelerate or delay the progress towards eliminating transmission of schistosomiasis in China? Infect Dis Pov 5(1):1–9
Zhu H-M, Xiang S, Yang K, Wu X-H, Zhou X-N (2008) Three Gorges Dam and its impact on the potential transmission of schistosomiasis in regions along the Yangtze River. EcoHealth 5:137–148
Zhu X, Xu Z, Liu Z, Liu M, Yin Z, Yin L, Zheng W (2022) Impact of dam construction on precipitation: a regional perspective. Mar Freshw Res. https://doi.org/10.1071/MF22135
Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM (2019) The essential metals for humans: a brief overview. J Inorg Biochem 195:120–129
Funding
This work was supported by the National Natural Science Foundation of China (51922065, 52279069) and Projects of the National Natural Science Foundation of China (52179018).
Author information
Authors and Affiliations
Contributions
All authors have an equal contribution.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical approval
Not applicable.
Consent of participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soomro, Seh., Shi, X., Guo, J. et al. Are global influences of cascade dams affecting river water temperature and fish ecology?. Appl Water Sci 13, 106 (2023). https://doi.org/10.1007/s13201-023-01902-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-023-01902-9