Abstract
Heavy metal has become a public health concern because of its tendency to accumulate in living organisms. The biosorptive removal of hexavalent chromium (Cr(VI)) from aqueous solution was investigated by using waste mosambi peel dust, and response surface methodology was applied as optimizing technique. The adsorbent was characterized by pHZPC, scanning electron microscope and Fourier transform infrared spectroscopy. The optimize condition for removal of Cr(VI) was recorded as follows: pH 2; dose: 0.5 g/50 mL; initial concentration: 5 mg/L; contact time: 30 min; agitation speed: 150 mg/L. The significance of independent variables and their interactions were tested by the analysis of variance and t test statistics. The adsorption data are nicely fitted with D–R isotherm and followed pseudo-second-order kinetic model. The monolayer adsorption capacity was recorded as 3.623 mg/g. Finally, thermodynamic results demonstrated that Cr(VI) adsorption is endothermic in nature and spontaneous. Therefore, the present results highlighted that mosambi peel dust is an efficient adsorbent and its versatility represents an eco-friendly alternative that can be implemented as agricultural waste management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The existence of heavy metal in the effluent is a tremendous problem for both terrestrial and aquatic community (Wang et al. 2011). There are number of industries such as tannery, leather, chrome plating, ceramics which discharge a significant amount of hexavalent chromium into the environment (Mutongo et al. 2014). When significant amount of chromium enter the human body, it may damage mainly kidney and liver for human being (Achmad et al. 2017). However, low level of chromium may also cause skin irritation, nose bleeding, respiratory track infection, etc. (Zhang 2009). The two main oxidation states of chromium are available in liquid medium; one is Cr(III) and other is Cr(VI). Moreover, out of these two oxidation state, Cr(VI) is thermodynamically more stable and highly mobile than Cr(III) (Mondal and Basu 2019; Ali et al. 2016). Although the form of Cr(VI) is different in different pH medium as chromate (Cr2O4), hydrochromate (HCrO4−) or dichromate (AL-Othman et al. 2012). Many techniques are available for removal of heavy metal an from aqueous solution such as ion exchange, electrodialysis, precipitation, adsorption, ultra-filtration, reverse osmosis; however, only adsorption technique is most suitable one because of its excellent performance and low operating cost (Mondal et al. 2019a; Mondal and Roy 2016).
Many low-cost materials have been used by the earlier researchers for removal of Cr(VI) from aqueous solutions such as Aspergillus niger (Mondal et al. 2017); banana peel (Ali et al. 2016); potato peel (Mutongo et al. 2014); human hair (Mondal and Basu 2019); groundnut hull (Owalude and Tella 2016); lychee peel (Rao et al. 2012); chicken feather (Mondal et al. 2019b), etc; however, none of the adsorbent showed high adsorption capacity. Therefore, a new easily available adsorbent should be developed for the fulfillment of the above objective.
In traditional optimization process, one independent variable change while maintaining other variables fixed which is time-consuming and laboratories take for a large number of independent variables. To overcome the above problem, response surface methodology (RSM) has been introduced for biosorption of hexavalent chromium from aqueous solution (Mondal et al. 2019b; Bhaumik and Mondal 2016).
In the present study, mosambi peel has been selected as a new low-cost biosorbent for the abatement of Cr(VI) from aqueous medium. Finally, the optimization of Cr(VI) removal was done by response surface methodology (RSM) using Design Expert Version 8.0.7.1. The experimental data were analyzed by fitting to a secondary polynomial model which again validated by analysis of variance (ANOVA) and lack-of-fit test.
Mosambi tree (Citrus limetta) is under the category of citrus species of the family Rutaceae (Yonis et al. 2015). It is native to the South Asia and Southeast Asia and also cultivated in the Mediterranean Basin. It is a small tree which may reach 8 m in height. It possesses numerous thrones throughout the tree. The skin of the tree is light yellow at maturity. The peels of mosambi have high pectin content which is a good antidote to blood sugar and cholesterol (Baker 1994). Moreover, mosambi peel contains many phytochemicals that are effectively used in pharmaceutical industry for drugs or food supplements (Middleton et al. 2000).
Materials and methods
Collection and preparation of mosambi peel
The mosambi peel was first washed thoroughly with deionized water. The soluble colored components of mosambi peel dust were then removed by washing repeatedly with hot deionized water. The peel was then dried at 70 °C for 24 h. The dried peel was then crushed, passed through a 50–300 sieve and stored in polythene bottles (Fig. 1).
Determination of pHZPC
The point of zero charge of the adsorbent was determined by the solid addition method. A 50 mL of 0.1 M KNO3 solution transferred into a series of 100 mL conical flask. The initial pH values of the solution were adjusted from 1.0 to 10.0 by adding either fixed strength of HNO3 (0.05, 0.1 and 0.5 N) or 0.1 N KOH. Then, 0.15 g of chicken feather, 1.5 g of mosambi peel and 1 g of human hairs were added to each flask which was securely capped immediately (Bhaumik et al. 2017). The flasks were then placed into a constant temperature water bath shaker and shaken for 24 h. The pH values of the supernatant liquid were noted after 24 h.
Sample preparation for SEM and FTIR study
After carrying out all the optimum experimental condition, adsorbents were filtered and dried at normal room temperature and finally analyzed their characteristics (Mondal and Kar 2018).
Metal solution preparation
An aqueous stock solution of chromium ions was prepared using potassium dichromate by dissolving 0.2828 g potassium dichromate (K2Cr2O7) in 250 mL of deionized water and dilute to 1 L in a volumetric flask with double-distilled water (100 mg/L). pH of the solution was adjusted using 0.1 N HCl or NaOH. Fresh dilutions were used for each sorption study.
Batch experiments for mosambi peel
Batch experiments were carried out in 100 mL conical flask containing 5 mg/L of chromium solution. 1.5 g of mosambi peel dry dust and 5 mg/L of 50 mL chromium solution were taken in each 250 mL conical flask. The desired pH of the respective solutions was maintained by adding 0.5 (N) HNO3 and/or 0.1 (N) NaOH. The contact time for each solution was maintained for 30 min. The stirring rate of the contact between solution and adsorbent was maintained at 150 mg/L; the particle size is 300 µ, and the temperature for all experiment, except temperature variation, was fixed at 40 °C.
Adsorption experiments were conducted in different batches where the pH, adsorbent dose, stirring rate, contact time, particle size, initial chromium concentration and temperature were changed. In these experiments, parametric ranges were done by changing pH from pH 1 to 10, adsorbent dose: 0.01 g to 1.5 g, stirring rate 150 mg/L to 350 mg/L, contact time: 1–40 min, particle size 50 µ to 300 µ, initial fluoride concentration: 5–30 mg/L and finally changes in temperature range from 30 to 55 °C. Influence of various operating parameters was studied by varying are parameter at a time and keeping the others constant. This is a serial adsorption process where the best removal of chromium for a parameter can be screening out and fixed the value of that parameter followed the next experiment. Then, the chromium was analyzed by spectrophotometer.
Metal analysis
Hexavalent chromium was form complex with 1–5 diphenyl carbazide, and final purple color was measured by UV spectrophotometer. The percentage of Cr(VI) removal was measured by using the following Eq. (1).
where cinitial and cfinal are the concentrations of Cr(VI) at the beginning and at the end of the adsorption process.
Model study
Various response surface methodologies are central composite; Box–Behnken and Doehlert have been extensively used in experimental design.
In the Box–Behnken design, total number of experiment can be calculated according to the following Eq. 2:
where k is the factors numbers, Cp is the replicate number of the central point.
This design has been used for fitting in a second-order polynomial Eq. 3:
where Y is the predicted response, b0 is the constant coefficient, This linear coefficient of the input factors Xi, bii is the liner interaction coefficient between the input factors Xi and Xj, and bij is the interaction coefficients of the input factors Xi (Sen et al. 2017).
Results and discussion
Adsorbent characterization
Mosambi peel dust showed very sharp bands at 3338 cm−1, 2918 cm−1, 2360 cm−1, 1604 cm−1. These bands attributed the functional groups such as alcoholic –O–H, –C–H, –P–H and –C=O stretching vibration (Fig. 2a). But after Cr(VI) adsorption, the active functional groups of the mosambi peel dust showed very nominal changes at 2360 cm−1, 1604 cm−1 and 1051 cm−1 and the changes in stretching frequency are 2362 cm−1, 1641 cm−1 and 1016 cm−1, respectively (Fig. 2b). This information clearly suggests that the functional groups –O–H, phosphoric –P–H and –C=O definitely form some kind covalent bonding with Cr(VI) ions (Saha et al. 2013).
SEM study
Scanning electron microscopy is a valuable technique to understand the surface morphology of the adsorbent. The present study results depicted that mosambi peel dust has numerous rough surface morphology (Fig. 3a) (Mondal and Roy 2018). However, after removal of Cr(VI), the surface of the mosambi peel dust is smooth enough which perhaps due to accumulation of Cr(VI) onto the surface of mosambi peel dust (Fig. 3b).
pHZPC study
Point of zero charge (pHZPC) is valuable parameter which helps to identify the availability of H+ or OH− ions on the surface of the adsorbent (Mushtaq et al. 2014). The present result depicted that mosambi peel has zero charge at pH 3.14 (Fig. 4). Therefore, removal of Cr(VI) is favorable below 3.14 pH, and the results of the present study also indicate that maximum Cr(VI) removal occurred at pH 1.0. This can be attributed by the fact that under such acidic condition chromium may exist as HCrO4− (Siboni et al. 2011). However, at alkaline pH, the dominant species is CrO42− and Cr2O72− which may repel by the negative surface. Almost similar observation was reported by Mondal et al. (2018).
Effect of initial concentration
In order to find out the best removal of Cr(VI) under the influence of different initial concentration of Cr(VI) fixed pH (2), dose (0.2 g), contact time (30 min), stirring rate (150 mg/L) and temperature (40 °C) was studied and it is presented in Fig. 1. Results revealed that percentage of Cr(VI) removal decreased from 94.6 to 22.11%, when initial concentration increased from 5 to 15 mg/L. However, after 15 mg/L, removal of Cr(VI) increases and reached to 45.89% at 30 mg/L (Fig. 5). From Fig. 5, it is also clear that adsorption capacity of the mosambi peel is 1.183 at higher percentage (94.6%) of the Cr(VI) removal. Almost similar variation of Cr(VI) removal by banana peel dust and orange peel dust was reported by earlier researcher (Pakshirajan et al. 2013).
Effect of adsorbents dose
Effect of adsorbent dose was carried out from 0.01 to 1.5 g under constant initial concentration (5 mg/L); pH (1); contact time (30 min) and temperature (40 °C). This indicates that percentage of Cr(VI) removal increases from 43.78 to 99.58% with increasing adsorbent dose from 0.01 to 0.5 g (Fig. 6). The percentage of Cr(VI) removal increased with increasing adsorbent dose is probably due to increase in surface area (Srividya and Mohanty 2009). However, adsorption capacity (mg/g) changes from 10.945 to 0.249 (mg/g) when percentage of removal gradually increased from 43.78 to 99.58% (Fig. 6). This can be attributed by the fact that freshly added adsorbent can make slight cover over some active sites.
Effect of pH
pH is a very important factor for adsorption of Cr(VI) from aqueous solution (Gupta et al. 2001). The present experiments were performed on 50 mL solution of 5 mg/L Cr(VI) and 0.2 g mosambi peel dust at 30 °C with varying pH from 1 to 10. The pH of the solution adjusted by using 0.1 (N) HCl or NaOH. The entire results are presented in Fig. 7. Study results revealed that removal of Cr(VI) gradually decreases with increasing pH of the solution from highly acidic to alkaline. Results indicate that 95.02% Cr(VI) was removed at pH 1. However, when pH of the solution changes to 4, 6, 8 and 10, the removal drastically changes to 41.92%, 40.96% and 29.7% and 13.26%, respectively. The higher removal of Cr(VI) at lower pH is possible due to electrostatics attraction between positive mosambi peel dust and chromium(VI) ions (Mondal et al. 2018). The metal biosorption can largely depend on the ionic functional groups available on the surface of the adsorbents and metal chemistry in the solution (Park et al. 2005). However, at higher pH, the entire surface of the adsorbents becomes negative and Cr(VI) removal decreased (Khambhaty et al. 2009). A possible interaction between Cr(VI) and main chemical constituents of mosambi peel is demonstrated in Figure S1.
Effect of contact time
In adsorption chemistry, it is important that the solution be allowed appropriate time to attain equilibrium (Srividya and Mohanty 2009). Figure 8 demonstrates the effect of various time interval on removal of Cr(VI) from aqueous solution, with constant of other variables such as initial concentration (5 mg/L), pH (1), adsorbent dose (0.5 g) and temperature (40 °C). Figure 8 clearly reveals that percentage of Cr(VI) removal increase with increasing contact time. However, throughout the experimental time period, there is a lack of consistency in removal of Cr(VI) by mosambi peel. Moreover, it was also noted that the rate of Cr(VI) binding with the mosambi peel is more at initial stages, which gradually decreases and become almost constant after an optimum period of 30 min. Almost similar finding was reported by (Mohanty et al. 2006).
Effect of temperature
The study of temperature on the adsorption study is extremely important for understanding the enthalpy and entropy changes during adsorption (Alkan and Dogan 2003). Moreover, temperature is the valuable indicator to understand the exothermic or endothermic nature of adsorption process (Salleh et al. 2011). The present study results are presented in Fig. 9. Figure 9 clearly indicates that Cr(VI) removal gradually increases with increasing temperature from 30 to 45 °C. However, after 45 °C, removal again decreases. Therefore, it can be suggested that the removal of Cr(VI) by mosambi peel is an endothermic process. Almost similar observation was reported by Mondal et al. (2019a, b) for removal of Cr(VI) from aqueous solution by chicken feather. The previous literature (Senthilkumar et al. 2006) also suggested that the enhancement at adsorption capacity at higher temperature is probably due to enhancement of adsorbent pore size and activation adsorbent surface.
Isotherm study for mosambi peel
On the basis of experimental results, biosorption isotherm was used to understand the interaction pattern of Cr(VI) with mosambi peel, at equilibrium (Chattoraj et al. 2018). The Langmuir model was based on the assumption that the adsorbate only attached to the specific sites of the adsorbent surface, suggesting that the uptake of absorbate is absolutely uni-layer without interaction between adsorbate molecules. The following form of Langmuir equation is traditionally applied (Eq. 4):
Equation (4) can conveniently transform to the following linearized form (Eq. 5):
where qm in the maximum uptake (mg/g), qe the uptake capacity at equilibrium (mg/g), Ce the equilibrium solution concentration (mg/L), b the Langmuir constant (L/mg). Figure 7 depicts the Cr(VI) adsorption by mosambi peel at constant temperature. The output of Langmuir constant (qm and b) and correlation coefficient (R2) is presented in Table 1. As can be seen from Table 4, the adsorption isotherm of Cr(VI) exhibited Langmuir behavior, which indicates a monolayer adsorption. The adsorption of Cr(VI) by human hair is well fitted with both Freundlich and Langmuir models; therefore, the Langmuir–Freundlich equation was also applied to the data sets. Basically, combined equation of Langmuir–Freundlich is known as Sips model. The Langmuir–Freundlich model was analyzed by Sips who observed that the energy distribution function corresponds to a symmetrical quasi-Gaussian function (Sips 1948). At lower concentration, Sips model changes to Freundlich model and in the case of a homogeneous surface, it reduces to the Langmuir model. The Langmuir–Freundlich equation can be expressed in the following way (Eq. 6):
This equation can be easily linearized as (Eq. 7):
Kinetics study for mosambi peel
To predict the kinetics of Cr(VI) adsorption by mosambi peel, pseudo-first-order (Lagergren), pseudo-second-order (Ho and Mekay 1999) and intraparticle diffusion kinetics models were used to fit the experimental data (Table 2). The values of qe and k2 can be determined from the slope and intercept of the plot (plot not shown) (Roy and Mondal 2017). From Table 2, it is clear that the experimental data well fitted with pseudo-second-order kinetics model with very high correlation coefficient (R2 = 0.997).
On the other hand, pseudo-first-order and intraparticle diffusion model are moderately fitted with the experimental data (Table 2). These results suggest the adsorption on to the adsorbent at specific temperature was best presented by the pseudo-second-order equation, which is based on the assumption that the rate-limiting step may be the chemisorption (Aksu 2001).
Thermodynamic study for mosambi peel
The thermodynamic parameters for the obtained equilibrium data on temperature variation by the use of Eqs. (8–9) were evaluated. The equilibrium constant Kc was calculated based on CAe and Ce values (Eq. 8):
where CAe indicates adsorption in mg/L at equilibrium and Ce is the equilibrium concentration of the metal in mg/L. The respective values of other thermodynamic parameters such as ∆H° and ∆S° were obtained from the slope, and interpret of the plot of log Kc against 1/T (Eq) revealed the values of free energy (∆G°) at different temperatures were obtained using Eq. 9.
where T is the temperature in Kelvin and R is the gas constant (kJ mol−1 K−1).
The entire results for the thermodynamic parameters are presented in Table 3. From Table 3, it is clear that both ∆H° and ∆S° is positive; the positive value of ∆H° for Cr(VI) removal by mosambi peel confirms that the adsorption process is endothermic in nature, and positive ∆S° indicates that the adsorption process is spontaneous (Ghosh and Mondal 2019). However, the spontaneity of chromium adsorption is also supported by the free energy value at different temperature (Table 3), according to the equation.
Modeling outcome
In the present study, Box–Behnken design was applied based on three levels four factors. The linear and second-order polynomial equation was fitted to the experimental data to obtain the regression equations. The various indicators such as lack-of-fit test, the sequential F test and other statistical tests were used for screen out the results such as sequential model sum of square, lack of fit and model summary statistics revealed that cubic model is aliased. The model is aliased, which means that the model is not sufficient for further investigation. However, model summery statistical of the mosambi peel adsorbents exhibited the goodness of fit is quite high (R2 = 0.980) compared to the quadratic model (R2 = 0.932) Table 4.
The model adequacy of the Cr(VI) adsorption by mosambi peel was assisted by different statistical test such as sequential model summary of square, lack of fit and model summary statistics and all the clearly demonstrate (p < 0.01) the said adsorption process is statistically viable. The second-order polynomial equations for Cr(VI) removal efficiency in terms of coded factors are given by Eq. 10;
Multiple regression analysis clearly suggests that the removal of Cr(VI) by mosambi peel is statistically viable. On the other hand, the adequacy of the model was assessed by ANOVA (Table 4). From Table 4, it was found that removal of Cr(VI) by mosambi peel is significant (p < 0.0001) with F value 13.76. The operating parameters such as initial concentration and pH of the Cr(VI) solution are statistically significant (p < 0.0001). However, the lack-of-fit F value (3.20) was recorded not significant relative to pure error. The adequate precision ratio is 12.217 which measures the signal to noise and as the value of adequacy precisions is > 4, therefore this model can be used to navigate the design space.
Judgment of model adequacy
The judgment of model can be done by the assumption to develop the model in such a way where the error in prediction is normally distributed. The difference between experimental value and predicted value is called residual value. The removal of Cr(VI) by mosambi peel can be presented by predicted removal versus residual removal of Cr(VI).
3D response surface plots
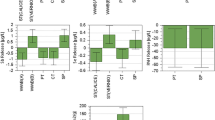
3D response surface plot is presented in Fig. 10a–f. Figure 10a–f represents the removal of Cr(VI) with variation of (a) pH and initial concentration (b) dose and initial concentration (c) contact time and initial concentration (d) dose and pH (e) contact time and pH and (f) contact time and dose. All these 3D plots show how the removal of Cr(V) simultaneously affected by two factors. Further, from 3D plot, it is clear that low pH and lower initial concentration are favorable for removal of Cr(VI) from aqueous solution (Das et al. 2013).
On the other hand, a normal probability plot and dot diagram of these residuals and actual versus predicted are presented in Fig. 11a, b. Figure 11a depicts that maximum data points are close to straight line leading to support to the conclusion that operating variables, interaction and square terms were significant. Similarly, Fig. 11b shows the relationship between the actual and predicted values for removal of Cr(VI) by mosambi peel. Again, it can be seen in Fig. 11b that the developed model is adequate due to residuals for the prediction of each response is minimum. The desirability plot of the chromium(VI) removal by mosambi peel clearly indicates that for achieving desirability 1.0, the operating variable should be fixed at initial concentration 6.75, pH 4.29, dose 0.27 g/100 mL and contact time 56.40 min (Fig. 12). Very recently, Mondal et al. (2019b) reported almost similar desirability value (1.0) with operating variables pH, initial concentration, contact time and adsorbent dose for removal of chromium (VI) by chicken feather.
Comparison of mosambi peel for Cr(VI) adsorption
The previous literature highlighted that Cr(VI) can be removed by various biosorbents such as materials including orange peel (Park et al. 2007), pine bark (Park et al. 2007), pine needles (park et al. 2007), rice bran (Sing et al. 2005), rice husk (Oliveira et al. 2005), sawdust (Sumathi et al. 2005; Park et al. 2005), sunflower stem (Jain et al. 2009), sugarcane bagasse (Garg et al. 2007), sugar beet pulp (Garg et al. 2007) and soya cake (Daneshvar et al. 2002) (Table 5). Hexavalent chromium adsorption capacity is usually determined by Langmuir and Freundlich adsorption isotherms but often calculated on different basis in different studies, such as mg/kg, mg/g or m mol/g for qe. A comparison of Cr(VI) adsorption capacity on with other of different reported adsorbents with mosambi peel has been presented in Table 5. It shows that the Cr(VI) adsorption capacity on mosambi peel is much higher than rice husk, sugar bagasse, soya cake and fly ash. However, other adsorbents such as orange peel, pine bark, rice bran, rice straw, fly ash and modified hectorite clays showed much higher Cr(VI) adsorption capacity than mosambi peel. Overall, all the three tested adsorbents exhibited the potential to be an efficient and low-cost adsorbent for Cr(VI) immobilization.
Conclusion
The equilibrium of Cr(VI) adsorption nicely fitted with Langmuir isotherm indicating a monolayer adsorption capacity of 1.1694 mg/g. Moreover, the kinetics of Cr(VI) adsorption in mosambi peel data showed a good agreement with pseudo-second-order which suggests that the rate-limiting step is controlled by chemical adsorption. On the other hand, thermodynamic study revealed that the adsorption of Cr(VI) by mosambi peel is endothermic reaction and entropy-driven process. The modeling results by the Box–Behnken model were used to screen out the influence of four operating variables on hexavalent chromium removal. Results demonstrated the nice fitting of second-order polynomial regression model with high R2 value of 0.932 with F value of 13.76. Therefore, the present study results concluded that use of mosambi peel power is a cost-effective method for the abatement of Cr(VI) from aqueous solution.
References
Achmad RT, Budiawan B, Auerkari EI (2017) Effects of chromium on human body. Ann Res Rev Biol 13(2):1–8
Agarwal GS, Bhuptawat HK, Chaudhari S (2006) Biosorption of aqueous chromium(VI) by Tamarindus indica seeds. Bioresource Technol 97(7):949–956
Aksu Z (2001) Equilibrium and kinetic modeling of chromium(II) biosorption by C. vulgaris in a batch system temperature: effect of temperature. Sep Purf Technol 21:285–294
Ali A, Saeed K, Mebood F (2016) Removal of chromium(VI) from aqueous medium using chemically modified banana peels as efficient low-cost adsorbent. Alex Eng J 55:2933–2942
Alkan M, Dogan M (2003) Adsorption kinetics of Victoria blue onto perlite. Fresenius Environ Bull 115:418–425
AL-Othman ZA, Ali R, Naushad Mu (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:224–238
Baker RA (1994) Potential dietary benefits of citrus pectin and fiber. Food Technol 48:13–138
Bhaumik R, Mondal NK (2016) Optimizing adsorption of fluoride from water by modified banana peel dust using response surface modelling approach. Appl Water Sci 6(2):115–135
Bhaumik R, Mondal NK, Chattoraj S (2017) An optimization study for defluoridation from synthetic fluoride solution using scale of Indian major carp Catla (Catla catla): an unconventional biosorbent. J Fluorine Chem 195:57–69
Chattoraj S, Mondal NK, Sen K (2018) Removal of carbaryl insecticide from aqueous solution using eggshell powder: a modeling study. Appl Water Sci 8:163. https://doi.org/10.1007/s13201-018-0808-5
Daneshvar N, Salari D, Aber S (2002) Chromium adsorption and Cr(VI) reduction to trivalent chromium in aqueous solutions by soya cake. J Hazard Mater 94(1):49–61
Das B, Mondal NK, Roy P, Chattoraj S (2013) Application of response surface methodology for hexavalent chromium adsorption onto alluvial soil of Indian origin. Int J Environ Pollut Solut 2:72–87. https://doi.org/10.7726/ijeps.2013.1007
Dehghani MH, SanaeiD Ali I, Bhatnagar A (2016) Removal of chromium(VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: kinetic modeling and isotherm studies. J Mol Liq 215:671–679
Elangovan R, Philip L, Chandraraj K (2008) Biosorption of chromium species by aquatic weeds: kinetics and mechanism studies. J Hazard Mater 152(1):100–112
Garg UK, Kaur MP, Garg VK, Sud D (2007) Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J Hazard Mater 140:60–66
Ghosh SB, Mondal NK (2019) Application of Taguchi method for optimizing the process parameters for the removal of fluoride by Al-impregnated Eucalyptus bark ash 11:100206. https://doi.org/10.1016/j.enmm.2018.100206
Gupta VK, Shrivastava AK, Jain N (2001) Biosorption of Chromium(VI) from aqueous solutions by green algae, Spirogyra species. Water Res 35:4079–4085
Jain M, Garg VK, Kadirvelu K (2009) Chromium(VI) removal from aqueous system using Helianthus annuus (sunflower) stem waste. J Hazard Mater 162(1):365–372
Ho YS, McKay G (1999) Pseudo-second order model for sorptionprocesses. Process Biochem. 34:451–465
Khambhaty Y, Mody K, Basw S, Jha B (2009) Biosorption of Cr(VI) onto marine Aspergillus niger: experimental studies and pseudo-second order kinetics. World J Micro Biotechnol 25:1413–1421
Kumar LL, Muthukumaran V, Gobinath MB (2009) Batch adsorption and kinetics of chromium(VI) removal from aqueous solutions by Ocimum americanum L. seed pods. J Hazard Mater 161(2–3):709–713
Middleton E, Kandaswami C, Theoarides TC (2000) The effects of plant flavanoids on mammalian cells: implications for information, heart disease, and cancer. Pharmacol Rev 52:673–751
Mondal NK, Basu S (2019) Potentiality of waste human hair towards removal of chromium(VI) from solution: kinetic and equilibrium studies. Appl Water Sci 9:49. https://doi.org/10.1007/s13201-019-0929-5
Mondal NK, Kar S (2018) Potentiality of banana peel for removal of Congo red dye from aqueous solution: isotherm, kinetics and thermodynamics studies. Appl Water Sci 8:157. https://doi.org/10.1007/s13201-018-0811-x
Mondal NK, Roy S (2016) Optimization study of adsorption parameters for removal of phenol on gastropod shell dust using response surface methodology. Clean Technol Environ Policy 18(2):429–447
Mondal NK, Roy A (2018) Potentiality of a fruit peel (banana peel) toward abatement of fluoride from synthetic and underground water samples collected from fluoride affected villages of Birbhum district. Appl Water Sci 8:90. https://doi.org/10.1007/s13201-018-0729-3
Mondal NK, Samanta A, Dutta S, Chattaraj S (2017) Optimization Cr(VI) biosorption onto Aspergillus niger using 3-level Box–Behnken design: equilibrium, kinetic, thermodynamic and regeneration studies. J Genet Eng Biotechnol. https://doi.org/10.1016/j.jgeb.2017.01.006
Mondal NK, Samanta A, Chakraborty S (2018) Shaikh WA (2018) Enhanced chromium(VI) removal using banana peel dust: isotherms, kinetics and thermodynamics study. Sustain Water Resour Manag 4:489–497. https://doi.org/10.1007/s40899-017-0130-7
Mondal NK, Ghosh P, Sen K, Mondal A, Debnath P (2019a) Efficacy of onion peel towards removal of nitrate from aqueous solution and field samples. Environ Nanotechnol Monit Manage 11:100222. https://doi.org/10.1016/j.enmm.2019.100222
Mondal NK, Basu S, Das B (2019b) Decontamination and optimization study of hexavalent chromium on modified chicken feather using response surface methodology. Appl Water Sci 9:50. https://doi.org/10.1007/s13201-019-0930-z
Mohanty K, Jha M, Meikap BC, Biswas MN (2006) Bisorption of Cr(VI) from aqueous solutions by Eichhornia crassipes. Chem Eng J 117:71
Mushtaq M, Tan IM, Ismail L, Nadeem M, Sagr M, Azam R, Hashmet R (2014) Influence of PZC (Point of Zero Charge) on the static adsorption of anionic surfactants on a Malaysian sandstone. J Dispers Sci Technol 35:343–349
Mutongo F, Kuipa O, Kuipa PK (2014) Removal of Cr(VI) from aqueous solutions using powder of potato peelings as a low cost sorbent. Bioinorg Chem Appl. https://doi.org/10.1155/2014/973153
Nemr AE (2009) Potential of pomegranate husk carbon for Cr(VI) removal from wastewater: kinetic and isotherm studies. J Hazard Mater 161(1):132–141
Oliveira EA, Montanher SF, Andrade AD, Nóbrega JA (2005) Rollemberg Equilibrium studies for the sorption of chromium and nickel from aqueous solutions using raw rice bran. Process Biochem 40:3485–3490
Owalude SO, Tella AC (2016) Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. Beni-Suef Univ J Basic Appl Sci. https://doi.org/10.1016/j.bjbas.2016.11.005
Pakshirajan K, Worku AN, Acheampong MA, Lubberding HJ, Lens PN (2013) Cr(III) and Cr(VI) removal from aqueous solutions by cheaply available fruit waste and algal biomass. Appl Biochem Biotechnol 170(3):498–513. https://doi.org/10.1007/s12010-013-0202-6
Park JM, Park D, Yun YS, Jo JH (2005) Mechanism of hexavalent chromium removal by dead fungal biomass of Aspergillus niger. Water Res 39:533–540
Park D, Lim S-R, Yun Y-S, Park JM (2007) Reliable evidences that the removal mechanism of hexavalent chromium by natural biomaterials is adsorption-coupled reduction. Chemosphere 70(2):298–305
Rao RAK, Rehman F, Kashifuddin M (2012) Removal of Cr(VI) from electroplating wastewater using fruit peel of Leechi (Litchi chinensis)Desal. Water Treat. 49:136–146
Roy TK, Mondal NK (2017) Biosorption of Congo Red from aqueous solution onto burned root Of Eichhornia crassipes biomass. Appl Water Sci 7:1841–1854. https://doi.org/10.1007/s13201-015-0358-z
Saha R, Mukherjee K, Saha I, Ghosh A, Ghosh SK, Saha B (2013) Removal of hexavalent chromium from water by adsorption on mosambi (Citrus limetta) peel. Res Chem Intermed 39:2245–2257
Salleh MAM, Mahmoud DK, Karim WAWA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280:1–13
Sen K, Mondal NK, Chattoraj S, Datta JK (2017) Statistical optimization study of adsorption parameters for the removal of glyphosate on forest soil using the response surface methodology. Environ Earth Sci 76(1):22. https://doi.org/10.1007/s12665-016-6333-7
Senthilkumar S, Kalaamani P, Porkodi K, Varadrajan PR (2006) Adsorption of dissolved from aqueous phase on to activated carbon prepared from agricultural waste. Bioresour Technol 97:1618–1625
Siboni MS, Samarghandi MR, Azizian S, Kim WG, Lee SM (2011) The removal of hexavalent chromium from aqueous solutions using modified holly sawdust: equilibrium and kinetics studies. Environ Eng Res 16(2):55–60
Sing KK, Rastogi RG, Hasan SH (2005) Removal of Cr(VI) from Wastewater Using Rice Bran. J Colloid Interface Sc 290(1):61–8
Sips R (1948) Combined form of Langmuir and Freundlich equations. J Chem Phys 16:490–495
Srividya K, Mohanty K (2009) Biosorption of hexavalent chromium from aqueous solutions by Catla catla scales: equilibrium and kinetics studies. Chem Eng J 155:666–673
Sun P, Liu Z-T, Liu Z-W (2009) Chemically modified chicken feather as sorbent for removing toxic chromium(VI) Ions. Ind Eng Chem Res 48(14):6882–6889
Wang XS, Li ZZ, Sun C (2008a) Removal of Cr(VI) from aqueous solutions by low-cost biosorbents: marine macroalgae and agricultural by-products. JHazard Mater 153:1176–1184
Wang XS, Li ZZ, Sun C (2008b) Removal of Cr(VI) from aqueous solutions by low-cost biosorbents: marine macroalgae and agricultural by-products. J Hazard Mater 153(3):1176–1184
Wang XS, Tmg YP, Tao SR (2008c) Removal of Cr(VI) from aqueous solutions by the nonliving biomass of alligator weed: kinetics and equilibrium. Adsorption 14:823–830
Wang Q, Seng J, Sui M (2011) Characteristics of adsorption, desorption and oxidation of Cr(III) on birnessite. Energy Procedia 5:1104–1108
Yonis K, Islam RU, Jahan K, Yousuf B, Roy A (2015) Effect of addition of mosambi (Citrus limetta) peel powder on textural and sensory properties of papaya jam. Cogent Food Agric 1:1023675
Zhang LS (2009) The advanced water treatment and reuse technology. Chemical Industry Press, Beijing
Acknowledgements
All authors express their sincere thanks to the all faculty members of the Department of Environmental Science, The University of Burdwan, Burdwan, for their both academic and moral support. Authors also extend their thanks to technical staff of University Instrumentation Centre for helping during taking scanning photographs.
Funding
The present research was funded by the University of Burdwan, West Bengal, in the form of M.Sc. dissertation work in the financial year 2011–2013 (M.Sc./Dis(5)/2011-13(5)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest in publishing the current paper.
Ethical approval
The research is based on effective and efficient use of waste material and the nature of waste is biological origin. Therefore, this particular research does not require any ethical approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mondal, N.K., Basu, S., Sen, K. et al. Potentiality of mosambi (Citrus limetta) peel dust toward removal of Cr(VI) from aqueous solution: an optimization study. Appl Water Sci 9, 116 (2019). https://doi.org/10.1007/s13201-019-0997-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-0997-6