Abstract
Natural as well as acid modified dead biomass of brown marine alga Sargassum sp. was employed for the elimination of cadmium and zinc ions from synthetic wastewater; batch mode experiments were carried out to optimize various factors like adsorbent dosage, contact time, pH, agitation speed and primary metal ions concentration at room temperature (298.15 K) for both types of adsorbents i.e. natural and acid treated. Application of Langmuir and Freundlich isotherms suggested that the modified biomass adsorbed better as compared to the natural one; though sorption on the natural biomass was a physical process while that on the modified one was a physico-chemical process and thus was relatively difficult. The quantity of cadmium ions adsorbed was greater than that of zinc ions. Adsorption equilibrium for the metal ions sorption on treated Sargassum sp. biomass was established within 60 min for both cadmium and zinc ions with 95.3 and 90.1% removal efficiencies, respectively, but it was greatly influenced by the pH of the solution. The optimal conditions in the batch experiments were as follows: cadmium ions were removed effectively using 0.5 g of adsorbent and 5 mg/L initial metal ions concentration at pH 4 and 150 rpm agitation speed whereas the best results for zinc ions were obtained with 1 g of adsorbent and 5 mg/L initial metal ions concentration at pH 3 and 200 rpm agitation speed. The experimental data fitted well to the pseudo first order model as the values of metal uptake capacities were in good agreement with the experimental values. Thermodynamic studies show that the process is spontaneous and endothermic in nature. Desorption and regeneration studies reveal that recovery of biosorbent is low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Provision of uncontaminated water to meet human needs has become a matter of great concern all over the world (Ramirez and Holmes 2008). A wide range of organic and inorganic contaminants are being produced from various industries and their presence in freshwater above the permissible limits strongly affects the quality of water and make it unfit for domestic use (Zaki and Hammam 2014). Substantial quantities of heavy metals are generated from different industries like mining, metallurgy and metal plating and thus have marked negative impact on the human health, ranging from metabolic disorder to chronic and carcinogenic effects, due to their non-biodegradability and persistence in ecosystem. In order to limit the exposure to these harmful contaminants, there is an urgent need to implement water treatment procedures for purifying industrial effluents from heavy metals up to acceptable limits before discharging into water bodies (Abdel-Ghani and El-Chaghaby 2014; Malakootian et al. 2009; Gupta et al. 2009; Kadirvelu and Namasivayam 2003). The usual complex compositions of wastewaters lead to different decontaminating processes for heavy metals involving techniques like chemical precipitation, coagulation, evaporation, extraction, membrane separation, reverse osmosis, solvent extraction, ion exchange, biological and electrochemical techniques; but these processes either engage high cost or are ineffective for elimination of heavy metals from less concentrated solutions (Mamatha et al. 2012). Moreover, applications of such conventional treatment procedures involve continuous consumption of chemicals which results in further environmental harms. Therefore, it is need of the day to find simple, low-price and safe cleaning methods for the removal of heavy metals from contaminated water. However, nanocomposites and bionanocomposites synthesized by sophisticated techniques and materials have also been emerging as novel adsorbent materials for the removal of heavy metals from wastewater (Rais and Imran 2015; Rais et al. 2017; Rais and Anam 2017a, b, c; Rais and Imran 2017a, b).
During the past few years, various natural adsorbents like Teak leaves and Pistachio shell carbon have come into view as eco-friendly and cost-effective options (Mohammad et al. 2011; Shaziya and Rais 2017). Similarly, natural adsorbents involving biological process i.e. biosorption have also obtained significant consideration because of their several benefits as compared to conventional practices (Ogata et al. 2014; Akpor and Muchie 2010). Biosorption is a simple process that operates under mild conditions and utilizes biomass and waste bio-products to eradicate heavy metals from aqueous solutions. Several kinds of algae, bacteria, fungi and agro-wastes having excellent binding capability for heavy metal ions are being employed as biosorbents. These natural biosorbents produce minute solid wastes that can be easily managed by incineration or landfill (Abdel-Ghani and El-Chaghaby 2014; Chopra and Pathak 2010). Researchers have employed both alive and metabolically inactive dead biomass for the elimination of heavy metals from wastewater and found it an innovative alternative technique for this purpose. Tomko et al. (2006) used fungal biomass for the removal of metals and concluded that 90–95% removal efficiencies can be achieved for copper, aluminum and antimony from their respective aqueous solutions. At present, most of the scientists are applying biomass pretreated with mineral acids, alkalis and salts in order to enhance its adsorption capacity. Javaid et al. (2011) employed pretreated mycelial biomass of fungus Aspergillus niger as adsorbent for the elimination of copper and nickel metals and the fungi treated with acids and sodium carbonate were found to solve the purpose quite efficiently. In a more recent study, pretreated fungal biomass was successfully employed as adsorbent for the removal of lead and nickel from single and binary metal systems (Rao and Bhargavi 2013).
Biological resources like marine algae (seaweeds) are easily accessible in many regions and are found in large quantities in the coastal areas of Singapore, Brazil, South America, Pakistan (Karachi coast) and India (Wynne 2003; Rizvi and Shameel 2004; Karthikeyan et al. 2007). Several investigations regarding metal adsorption by brown algae have been carried out and high adsorption efficiencies for nickel, copper, chromium, cadmium, mercury, zinc, iron and cobalt have been reported (Saravanan et al. 2011; Vijayaraghavan et al. 2005; Padilha et al. 2005; Cossich et al. 2002). It is believed that the adsorption potential of these species mainly depends on their cellular structure and number of binding sites (Fourest and Volesky 1996) and also, on biomass dosage, concentration of metal ions and pH of the aqueous solution (Das et al. 2008; Davis et al. 2000). Studies reveal that Sargassum sp. is the best among natural biosorbents because of its significant number of binding sites (Yang and Volesky 1999; Cossich et al. 2002; Davis et al. 2004).
The present study was carried out to determine the adsorption potential of dead biomass of Sargassum sp., brown marine alga, for the removal of heavy metals i.e. cadmium (Cd) and zinc (Zn) from synthetic wastewater. The adsorbent was used in its natural as well as chemically treated form and both the adsorbents were characterized by Fourier transform infrared spectroscopy. To identify the optimum conditions for the elimination of heavy metal ions, effect of various factors i.e. biosorbent dosage, pH, contact time, preliminary metal ion concentration and agitation speed was studied through batch experiments. Langmuir and Freundlich models were applied to the batch experiments data and pseudo-first and second-order reactions were used to realize the kinetics of the adsorption process.
Materials and methods
Preparation of biosorbent
One kilogram sample of dead biomass of Sargassum sp. brown alga was arranged from Blunji area of Karachi coast. The sample was thoroughly washed with distilled water to eradicate any grit, litter and surplus material and was dried in an electric oven at (80 ± 2) °C for 24 h. The moisture free biomass was chopped manually to cut down into small pieces. It was then ground to fine powder with the help of mechanical grinder to pass through 200 mesh sieve, which was employed for further experimental work.
Modification of biosorbent
A part of the powdered natural biosorbent was subjected to acid pretreatment. 50 g of the biomass was soaked in 500 mL of 0.1 N HCl and the solution was stirred for 4 h at room temperature with the help of mechanical stirrer. The biosorbent was filtered and was extensively washed with distilled water in order to get neutral product and was dried in an electric oven at (80 ± 2) °C for 24 h.
Characterization of biosorbent
Both natural and pretreated samples of the biosorbent were characterized by Fourier transform infrared spectroscopy using Thermo Nicolet IR 200 (USA).
Preparation of standard solutions
1000 ppm stock solutions of cadmium and zinc were prepared by dissolving appropriate quantities of their respective chlorides in distilled water and working standards of required strength were prepared by diluting the stock solutions.
Biosorption experiments
Batch experiments were conducted to optimize the adsorption parameters like adsorbent dosage, contact time, pH of the medium, initial concentration of metal ions and agitation speed for both natural and treated biosorbents. The effect of these variables was investigated by varying adsorbent dosage (0.1, 0.3, 0.5, 1.0 and 1.5 g), contact time (15, 30, 35, 60, 75 and 90 min), pH (ranging from 2 to 8), initial concentration (5, 10, 15, 20 and 25 mg/L) and agitation speed (50, 100, 150, 200 and 250 rpm). In each experiment for both cadmium and zinc metal ions, weighed quantity of adsorbent was added to a specific volume of standard solution and after adjusting the desired pH, the mixture was agitated at invariable speed at room temperature keeping contact time constant. The solution thus obtained was filtered through Whatman filter paper No. 40. The residual metal ion concentrations of both cadmium and zinc were determined in the filtrate solutions by atomic absorption spectrometer (Hitachi Z-8000) and the percentage removal of metal from the solution was calculated using the formula:
where C i is the initial metal ion concentration in milligrams per liter and C f is the final metal ion concentration in milligrams per liter (Mamatha et al. 2012).
Similarly, the adsorption capacity, q was calculated for each experiment in milligrams per gram according to the formula:
where C i is the initial metal ion concentration in milligrams per liter; C f is the final or equilibrium metal ion concentration in milligrams per liter; V is the volume of metal ion solution in L and M is dry mass of the biosorbent in grams (Cossich et al. 2002).
Results and discussion
Characterization of biosorbents by Fourier transform infrared spectroscopy
The FT-IR spectra of both natural and treated biosorbents were recorded in the range of 4000–400 cm−1 and are presented in Fig. 1a, b. The spectra identify the surface functional groups and also illustrate the changes in biosorbent after acid treatment. The spectrum for natural Sargassum sp. exhibits several bands of multiple intensities merged to form a broad and strong band in the functional group region between 3200 and 3600 cm−1, associated with the stretching vibrations of free or bonded OH groups of alcohols and carboxylic acids and also with the presence of inter and intramolecular hydrogen bonding between these hydroxyl groups (Bojić et al. 2013). Generally, the stretching vibrations due to –NH group overlapped by the absorption bands of hydroxyl groups also appear in this range (Liu et al. 2011). The band of minor intensity at 2929.48 cm−1 corresponds to the asymmetric stretching vibrations of alkyl chains whereas that of medium intensity at 1635.50 cm−1 appeared due to C=O stretching vibrations (Kirova et al. 2012). The relatively small absorption bands at 1422.51, 1254.34 and 1031.70 cm−1 are also assigned to the stretching vibrations of COOH and OH groups (Kirova et al. 2012; Liu et al. 2011; Kleinübing et al. 2010). The broad band below 1000 cm−1 indicates the presence of long alkyl chain (Kirova et al. 2012). It can be observed that after acid treatment, the absorption bands appear at similar positions but with less intensities which shows that acid treatment had a significant effect on both the functional groups. FT-IR results suggest that hydroxyl and carboxyl groups are present on the surface of both natural and acid modified biosorbents that are believed to be responsible for metal biosorption (Kirova et al. 2012).
SEM analysis
In order to predict change in morphology of the biosorbent the SEM spectra of the natural and treated material have been taken at 15.0 kV × 1.0 k and 15.0 kV × 500 resolutions and shown in Fig. 2a, b. The SEM images show that porous nature of the material provide potential sites from inner cavities Fig. 2a. After acid treatment small channels and hollow cylindrical shaped pores are developed which is attributed to the evolution of trapped gasses from the dead biomass Fig. 2b.
Effect of biosorbent dosage
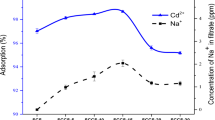
A series of experiments was conducted to investigate the effect of dosage of both natural and treated biosorbents on the biosorption of cadmium and zinc at five different dosages i.e. 0.1, 0.3, 0.5, 1.0 and 1.5 g, keeping contact time 60 min, pH 4, initial metal ion concentration 10 mg/L and agitation speed 150 rpm. It was found that in case of Cd biosorption, as the biosorbent dosage increases from 0.1 to 0.3 g, a gradual increase in the biosorption efficiency occurs followed by a significant increase in metal uptake at 0.5 g dose i.e. 71.9%; further increase in the quantity of biosorbent leads to consistent metal removal with a minor change, as a result of which equilibrium is established (Fig. 2). So, 0.5 g of natural biosorbent was found to be sufficient for removing a considerable quantity of Cd. Similarly, when acid treated biosorbent was used, the same dose removed 95.3% of cadmium and equilibrium is accomplished since further increase in biosorbent dosage resulted in a negligible change in the metal uptake. For zinc biosorption, the figure shows that 1 g each of natural and acid treated biosorbent was enough to remove 76.7 and 91.1% Zn, respectively. Therefore, it can be said that although natural biosorbent removed substantial quantities of both cadmium and zinc metals but quite enhanced results were obtained for biosorbent after acid modification.
Effect of contact time
Biosorption of Cd and Zn on natural and treated biosorbents was studied as a function of contact time and equilibrium time was determined for maximum uptake of metals. In a series of experiments, the contact time was varied from 15 to 90 min while all other parameters were kept constant and results are shown in Fig. 3. It can be seen that biosorption of Cd for both natural and treated biosorbents attained equilibrium after 60 min contact time with 71.5 and 95.3% removal efficiencies, respectively. The figure clearly shows that metal uptake by both the biosorbents was slow within first 30 min that increased abruptly in the next 15 min and reached 48.1% removal efficiency. As the contact time exceeds from 45 to 60 min, again a rapid increase in metal uptake was observed and equilibrium was attained. Therefore, further increase in contact time showed no substantial improvement in biosorption of Cd. The plot of contact time versus adsorption efficiency for biosorption of Zn on natural biosorbent confirms similar behavior like cadmium since equilibrium was established after 60 min contact time for natural as well as treated biosorbents with 76.4 and 90.1% removal efficiencies, respectively.
Effect of pH
Experiments were performed to study the effect of pH varied from 2 to 8, on the biosorption efficiency of Cd and Zn metals and the results are presented in Fig. 4. The figure shows that as the pH was increased, the uptake of Cd by both the biosorbents showed a regular increase till a maximum biosorption was reached at pH 4 and appreciable quantities of metal were adsorbed by natural and treated biomass i.e. 72.3 and 95.5%, respectively. However, further increase in pH resulted in a gradual decrease in the quantity of metal biosorbed and as the pH reached 8, the metal uptake reduced to an insignificant level i.e. 18.6 and 21.3% for natural and treated biosorbents, respectively. Likewise, the plots for zinc biosorption showed the same trend with the maximum sorption at pH 3 in case of both the biosorbents. These results show that less acidic conditions facilitate the biosorption of Cd and Zn metals on natural as well as acid modified Sargassum sp. sorbents since increase in pH towards basic conditions leads to a considerable decrease in biosorption. Consequent upon these results, it can be said that highly acidic conditions may reduce the surface hydroxyl groups responsible for the adsorption process; alkaline pH on the other hand, leads to a significant increase in concentration of hydroxyl groups in the solution, as a result of which affinity of hydroxyl groups for cadmium and zinc ions increases in the solution as compared to those present on the surface of adsorbent.
Effect of initial metal ions concentration
The effect of initial concentration of metal ions was studied at different concentrations of the adsorbate while unvarying all the other process parameters. Figure 5 indicates the adsorption behavior of Cd and Zn for both natural and treated biosorbents. It was noted that sorption efficiency is inversely proportional to the metal ions concentration i.e. increasing the metal ions concentration decreases the sorption efficiency. The maximum adsorption of cadmium was observed at 5 mg/L i.e. 95.80 and 98.40% removal efficiencies for natural and acid treated biosorbents, respectively. A gradual decrease in the sorption efficiency occurred as the concentration of metal ions was increased from 10 to 25 mg/L. In case of Zn when natural adsorbent was used, 85.20% adsorption efficiency was observed at 5 mg/L metal ions concentration and unlike to Cd, there was a slight increase in adsorption efficiency when concentration of zinc ions was increased to 10 mg/L i.e. 86.70% but as the concentration was increased to 15 mg/L a prominent decrease in adsorption efficiency took place, followed by a gradual decrease in adsorption at 20 and 25 mg/L. On the other hand, for acid modified sorbent similar behavior like Cd was noticed and maximum removal efficiency i.e. 94.20% was obtained at 5 mg/L initial zinc ions concentration.
Effect of agitation speed
Adsorption studies were carried out to study the effect of agitation speed on the sorption efficiencies of Cd and Zn metals using natural and acid treated biosorbents. The agitation speed was varied from 50 to 250 rpm whereas other process parameters were kept constant as optimized in above experiments. The results of experiments for both the metals are illustrated in Fig. 6 which clearly shows that no significant adsorption took place at 50 rpm, but as the agitation speed was increased to 100 rpm, a rapid increase in removal efficiency of Cd was noticed. At 150 rpm, a gradual increase in removal efficiency occurred i.e. 71.8 and 95.5% for natural and treated biosorbents; that led to equilibrium at 200 and 250 rpm agitation speeds. In the same way, the adsorption of Zn metal increased rapidly as the agitation speed increased from 50 to 200 rpm and a stage of equilibrium was attained at this speed with 76.7 and 90.5% removal efficiencies in case of both natural and modified adsorbents, respectively.
Application of adsorption isotherms
The equilibrium between the solid and liquid phases was studied using Langmuir and Freundlich models.
Langmuir isotherm
This model applies to the adsorption of solute with a homogeneous distribution of binding sites and equivalent adsorption energies including no interaction between the molecules of the solute and a point is reached where maximum adsorption takes place and finally equilibrium is attained (Ogata et al. 2014). The Langmuir isotherm is given by the following equation:
where q e is the amount of sorbate biosorbed at equilibrium in milligrams per gram; C e is the equilibrium concentration of the sorbate or the unabsorbed sorbate in the solution in milligrams per liter; b is the Langmuir isotherm constant that measures the biosorption energy and q o is the maximum theoretical biosorption capacity in milligrams per gram. Values of b and q o were calculated from the graph between C e/q e and C e. The dimensionless separation factor, R L that specifies the shape of the Langmuir isotherm and predicts the feasibility of adsorption process is expressed as:
When R L > 1 the adsorption process is unfavorable; when R L = 1, it is linear; 0 < R L < 1, it is favorable; when R L = 0, it is irreversible (Kadirvelu and Namasivayam 2003).
Figure 7 shows the adsorption isotherms for cadmium and zinc adsorbed on natural and acid treated biosorbents. The experimental data for the adsorption isotherms was applied to the above equations and their respective Langmuir constants are shown in Table 1. It can be seen that for cadmium, the correlation coefficient of the Langmuir constant i.e. R 2 is 0.792 for natural adsorbent (Fig. 7a). On the other hand, the correlation coefficient for the modified adsorbent is 0.983 (Fig. 7b). The table shows that the values of R L for cadmium adsorption on both natural and treated absorbent were greater than 0 and less than 1 (0 < R L < 1). Similarly, the values of correlation coefficient for zinc adsorption were calculated as 0.8722 and 0.9937 for natural and modified biosorbents, respectively (Fig. 7c, d), whereas R L was found to be greater than 0 and less than 1 in both the cases. These results suggest that adsorption of cadmium and zinc ions were found to be more favorable in case of acid treated biosorbent as compared to the natural one.
Freundlich isotherm
Freundlich model is based on the sorption of solute on a heterogeneous surface and involves the interaction between the molecules of the adsorbate. This model applies well to the non-ideal as well as multilayer adsorption but gives no information regarding the monolayer adsorption capacity, unlike the Langmuir model. The Freundlich isotherm equation is expressed as (Ogata et al. 2014):
where q e (q t) is the sorbate biosorbed at the equilibrium in milligrams per gram; C e is the equilibrium concentration of the sorbate or the unabsorbed sorbate in the solution in milligrams per liter; K F is the Freundlich constant that relates to the biosorption capacity. Values of K F and n were evaluated by plotting a graph between logq t and logC e; n is the constant that represents the measure of nonlinearity between concentration of metal ions and adsorption; adsorption is linear when n = 1; for n < 1, adsorption is a chemical process; adsorption is a physical process for n > 1 (Abdel-Ghani and El-Chaghaby 2014).
The adsorption isotherms for cadmium and zinc ions on natural and acid treated biosorbents are illustrated in Fig. 8a–d.
The Freundlich model was applied to the experimental data for these adsorption isotherms and values of related constants are shown in Table 1. It can be noticed that the values of correlation coefficient (R 2) for cadmium adsorption were calculated to be 0.859 and 0.957 on natural and acid treated biosorbents, respectively. The table shows the values of K F i.e. 0.711 and 0.065 for cadmium against natural and treated biomass, respectively. Since, the values of K F are less than 1 therefore, the adsorption process is considered to be favorable (Schwarzenbach et al. 2003). The values of n were measured to be 3.30 and 0.26 for Cd natural and treated biosorbent, respectively, that indicate that adsorption is a physical process in case of natural biosorbent, on the other hand, it is a chemical process for the modified biosorbent. The Freundlich constant (K F) was found to be 0.60 and 1.44 for zinc against natural and treated biomass, respectively, whereas the values of n were calculated as 3.42 and 0.43 for natural and treated biosorbents, respectively. The values of n show that zinc ions were adsorbed physically on natural biosorbent but chemically on the treated one. The values of correlation coefficient (R 2) for zinc adsorption were calculated to be 0.748 and 0.966 on natural and acid treated biosorbents, respectively. From these results it can be concluded that adsorption of cadmium and zinc ions is more favorable on treated biosorbent than untreated natural biosorbent.
Adsorption kinetics
Pseudo first order rate kinetics
The Lagergren’s pseudo-first-order kinetic model is based on the binding of each metal ion to only one sorption site on the surface of the adsorbent. In this model, the rate biosorption sites are occupied is proportional to the number of unoccupied sites (Abdel-Ghani and El-Chaghaby 2014). The Lagergren’s rate equation is expressed as (Kadirvelu and Namasivayam 2003):
where q e and q are the sorbate adsorbed in milligrams per gram at equilibrium and at time t, respectively, and K 1 is the first order rate constant (g/mg/min).
The rate kinetics was determined by plotting log(q e − qt) against time (t); values of K 1 and q e were calculated from the slope and intercepts of the corresponding plots. Figure 9 shows the plots of pseudo first order rate kinetics for Cd and Zn ions on natural as well as treated adsorbents and the relevant rate constants values are shown in Table 2. The table shows the experimental as well as calculated q e values for both the metals appear to be quite closer to each other, thus indicating that the adsorption processes follow pseudo first order reaction kinetics.
Pseudo second order rate kinetics
The pseudo-second-order rate equation is given as:
If the process follows second-order kinetics, then graph between t/q and t should be a straight line; q e and K 2 can be calculated from its slope and intercept, respectively (Abdel-Ghani and El-Chaghaby 2014).
Figure 10 gives the graphical representation of pseudo second order rate kinetics of Cd and Zn for both types of adsorbents and the related rate constants are shown in Table 2. The correlation coefficients obtained for Cd were 0.443 and 0.549 in case of natural and treated biosorbents, respectively; similar values were obtained for Zn. The table shows the experimental and theoretically calculated values of q e for both the metals differ greatly. These results suggest that the adsorption processes do not follow pseudo second order kinetics since the values of correlation coefficients are significantly less than 1 and also the experimental and calculated q e values are not in good agreement with each other (Table 3).
Thermodynamic studies
Standard Enthalpy change ΔH 0, entropy change ΔS 0 and Gibbs free energy change ΔG 0 addressing the adsorption of Cd2+ and Zn2+ on treated biomass of Sargassum sp. have been determined. Data have been plotted between lnk and 1/T (Fig. 11).The values of ΔH 0 and ΔS 0 have been calculated from slope and intercept of the graphs and expressed in Table 4. The values for thermodynamic parameters indicate that the process is endothermic in nature and adsorption process is spontaneous and feasible (Vadivelan and Kumar 2005).
Metal desorption
Metal desorption studies of Cd(II) and Zn(II) at different acid concentration of HNO3 have been determined and findings are shown in Fig. 12. The results show that Cd(II) desorption was maximum i.e. 36.3% at 0.3 N acid concentration while Zn(II) desorption was maximum i.e. 21.6% at 0.3 N. Desorption process seems to be economical although recovery and regeneration capacity of the biosorbent has been low (Fig. 13).
Conclusion
The Sargassum sp. is a natural biosorbent abundantly available at coastal areas. From the present research work it can be concluded that modified Sargassum sp. biomass is a very good low cost and environment friendly biosorbent for decontaminating wastewater containing heavy metals. The studies may be extended for the elimination of organic pollutants as well.
References
Abdel-Ghani NT, El-Chaghaby GA (2014) Biosorption for metal ions removal from aqueous solutions: a review of recent studies. Int J Latest Res Sci Technol 3(1):24–42
Akpor OB, Muchie M (2010) Review: remediation of heavy metals in drinking water and wastewater treatment systems: processes and applications. Int J Phys Sci 5(12):1807–1817
Brown P, Atyl Jefcoat I, Parrish D, Gill S, Graham E (2007) Evolution of the adsorptive capacity of peanut hull pellets for heavy metals in solution. Adv Environ Res 4:19–29
Bojić DV, Ranđelović MS, Zarubica AR, Mitrović JZ, Radović MD, Purenović MM, Bojić AL (2013) Comparison of new biosorbents based on chemically modified Lagenaria vulgaris shell. Desalin Water Treat. doi:10.1080/19443994.2013.771287
Chopra AK, Pathak C (2010) Biosorption technology for removal of metallic pollutants-An overview. J Appl Nat Sci 2(2):318–329
Cossich ES, Tavares CRG, Ravagnani TMK (2002) Biosorption of chromium(III) by Sargassum sp. Biomass Electron J Biotechnol 5(2):133–140
Das N, Vimala R, Karthika P (2008) Biosorption of heavy metals—an overview. Indian J Biotechnol 7:159–169
Davis TA, Volesky B, Vieira RHSF (2000) Sargassum seaweed as biosorbent for heavy metals. Water Res 34(17):4270–4278
Davis TA, Ramirez M, Mucci A, Larsen B (2004) Extraction, isolation and cadmium binding of alginate from Sargassum sp. J Appl Phycol 16:275–284
Fourest E, Volesky B (1996) Contribution of sulfonate groups and alginate to heavy metal biosorption by the dry biomass of Sargassum fluitans. Environ Sci Technol 30:277–282
Ganji MT, Khosravi M, Rakhasaee R (2005) Biosorption of Pb, Cd, Cu and Zn from wastewater by treated Azolla filiculoides with H2O2/MgCl2. Int J Environ Sci Technol 1:265–271
Gupta BS, Curran M, Hassan S, Ghosh TK (2009) Adsorption characteristics of Cu and Ni on Irish peat moss. J Environ Manag 90:954–960
Ibrahim KM, NasserED-deen T, Khoury H (2002) Use of natural chabazite–phillipsite tuff in wastewater treatment from electroplating factories in Jordan. Environ Geology 41:547–551
Javaid A, Bajwa R, Manzoor T (2011) Biosorption of heavy metals by pretreated biomass of Aspergillus niger. Pak J Bot 43(1):419–425
Junior OK, Gurgel LVA, deMelo JCP, Botaro VR, Melo TMS, deFreitas Gil RP, Gil LF (2006) Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse. Bioresour Technol 98:1291–1297
Kadirvelu K, Namasivayam C (2003) Activated carbon from coconut coirpith as metal adsorbent: adsorption of Cd (II) from aqueous solution. Adv Environ Res 7:471–478
Karthikeyan R, Vijayalakshmi S, Balasubramanian T (2007) Monthly variations of heavy metals and metal resistant bacteria from the Uppanar estuary (Southeast coast of India). Res J Microbiol 2:50–57
Kirova G, Velkova Z, Gochev V (2012) Copper (II) removal by heat inactivated Streptomyces fradiae biomass: surface chemistry characterization of the biosorbent. J BioSci Biotech 77–82 (SE/ONLINE)
Kleinübing SJ, Vieira RS, Beppu MM, Guibal E, Carlos da Silva MG (2010) Characterization and evaluation of copper and nickel biosorption on acidic algae Sargassum filipendula. Mater Res 13(4):541–550
Liu H, Yang F, Zheng Y, Kang J, Qu J, Chen JP (2011) Improvement of metal adsorption onto chitosan/Sargassum sp. composite sorbent by an innovative ion-imprint technology. Water Res 45:145–154
Mahmood Z, Amin A, Zafar U, Raza MA, Hafeez I, Akram A (2017) Adsorption studies of cadmium ions on alginate-calcium carbonate composite beads. Appl water Sci 7(2):915–921
Malakootian M, Nouri J, Hossaini H (2009) Removal of heavy metals from paint industry’s wastewater using Leca as an available adsorbent. Int J Environ Sci Technol 6(2):183–190
Mamatha M, Aravinda HB, Puttaiah ET, Manjappa S (2012) Adsorption of ferrous and ferric ions in aqueous and industrial effluent onto Pongamia pinnata tree bark. Int J Chem Biomol Metall Mater Sci Eng 6(7):49–57
Mohammad A, Rifaqat AKR, Rais A (2011) Adsorption studies of heavy metals on Tectona grandis: removal and recovery of Zn (II) from electroplating wastes. J Disper Sci Technol 32:851–856
Nabizadeh R, Naddafi K, Saeedi R (2006) Biosorption of Pb(II) and Cd(II) from aqueous solutions by protonated sargassum sp. Biomass 5(1):21–26
Nasernejad B, Zadeh TE, Pour BB, Bygi ME, Zamani A (2005) Comparison for biosorption modeling of heavy metals Cr(III), Cu(II), Zn(II) adsorption from wastewater by carrot residues. Process Biochem 40:1319–1322
Ogata F, Kangawa M, Iwata Y, Ueda A, Tanaka Y, Kawasaki N (2014) A study on the adsorption of heavy metals by using raw wheat bran bioadsorbent in aqueous solution phase. Chem Pharm Bull 62(3):247–253
Ouki SK, Kavannagh M (1997) Performance of natural zeolites for the treatment of mixed metal-contaminated effluent. Waste Manag Res 15:384–494
Padilha FP, De França FP, Costa DA, Antonio CA (2005) The use of waste biomass of Sargassum sp. for the biosorption of copper from simulated semiconductor effluents. Bioresour Technol 96:1511–1517
Pehlivan E, Cetin S, Yanik BH (2006) Equilibrium studies for the sorption of zinc and copper from aqueous solutions using sugar beet pulp and fly ash. J Hazard Mater 135:193–199
Rais A, Anam M (2017a) Heavy metal remediation by dextrin-oxalic acid/cetyltrimethyl ammonium bromide (CTAB)–montmorillonite (MMT) nanocomposite. Groundw Sustain Dev 4:57–65
Rais A, Anam M (2017b) Adsorption of Pb(II) and Cu(II) by alginate-Au–mica bionanocomposite: kinetic, isotherm and thermodynamic studies. Proc Saf Environ Protect 109:1–10
Rais A, Anam M (2017c) Inulin-folic acid/bentonite: a novel nanocomposite for confiscation of Cu(II) from synthetic and industrial wastewater. J Mol Liq 241:489–499
Rais A, Imran H (2015) l-Cystein modified bentonite–cellulose nanocomposite (cellu/cys-bent) for adsorption of Cu2+, Pb2+, and Cd2+ ions from aqueous solution. Sep Sci Technol. doi:10.1080/01496395.2015.1095211
Rais A, Imran H (2017a) Efficient remediation of an aquatic environment contaminated by Cr(VI) and 2,4-dinitrophenol by XG-g-polyaniline@ZnO nanocomposite. J Chem Eng Data 62(5):1594–1607
Rais A, Imran H (2017b) l-Methionine montmorillonite encapsulated guar gum-g-polyacrylonitrile copolymer hybrid nanocomposite for removal of heavy metals. Groundw Sustain Dev 5:75–84
Rais A, Imran H, Alok M (2017) Adsorption of Cr (VI) and Cd(II) on chitosan grafted polyaniline–OMMT nanocomposite: isotherms, kinetics and thermodynamics studies. Desalin Water Treat 58:144–153
Ramirez OH, Holmes SM (2008) Novel and modified materials for wastewater treatment applications. J Mater Chem 18:2751–2761
Rao PR, Bhargavi C (2013) Studies on biosorption of heavy metals using pretreated biomass of fungal species. Int J Chem Chem Eng 3(3):171–180
Rizvi MA, Shameel M (2004) Studies on the bioactivity and elementology of marine algae from the coast of Karachi, Pakistan. Phytother Res 18:865–872
Saravanan A, Brindha V, Krishnan S (2011) Studies on the structural changes of the biomass Sargassum sp. on metal adsorption. J Adv Bioinform Appl Res 3(2):193–196
Schwarzenbach RP, Gschwen MP, Imboden DM (2003) Environmental organic chemistry. John Wiley & Sons Inc., Hoboken
Shaziya H, Rais A (2017) Pistachio shell carbon (PSC) an agricultural adsorbent for the removal of Pb(II) from aqueous solution. Groundw Sustain Dev 4:42–48
Suzuki Y, Kametani T, Maruyama T (2005) Removal of heavy metals from aqueous solution by non-living ulva rigida. J Sci Food Agri 99:445–449
Tomko J, Bačkor M, Štofko M (2006) Biosorption of heavy metals by dry fungi biomass. Acta Metall Slovaka 12:447–451
Vadivelan V, Kumar KV (2005) Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J Colloid Interface Sci 286:90–100
Vijayaraghavan K, Jegan J, Palanivelu K, Velan M (2005) Biosorption of cobalt(II) and nickel(II) by seaweeds: batch and column studies. Sep Purif Technol 44:53–59
Wynne MJ (2003) Leveillea major sp. nov. (Rhodomelaceae, Rhodophyta) from the Sultanate of Oman. Bot Mar 46:357–365
Yang J, Volesky B (1999) Biosorption of uranium on Sargassum biomass. Water Res 33(15):3357–3363
Zaki MS, Hammam AM (2014) Aquatic pollutants and bioremediations [review]. Life Sci J 11(2):362–369
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mahmood, Z., Zahra, S., Iqbal, M. et al. Comparative study of natural and modified biomass of Sargassum sp. for removal of Cd2+ and Zn2+ from wastewater. Appl Water Sci 7, 3469–3481 (2017). https://doi.org/10.1007/s13201-017-0624-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0624-3