Abstract
Preparation and characterization of raw and activated carbon derived from three different selected agricultural wastes: kola nut pod raw and activated (KNPR and KNPA), bean husk raw and activated (BHR and BHA) and coconut husk raw and activated (CHR and CHA) were investigated, respectively. Influences of carbonization and acid activation on the activated carbon were investigated using SEM, FTIR, EDX, pHpzc and Boehm titration techniques, respectively. Carbonization was done at 350 °C for 2 h followed by activation with 0.3 M H3PO4 (ortho-phosphoric acid). Results obtained from SEM, FTIR, and EDX revealed that, carbonization followed by acid activation had a significant influence on morphology and elemental composition of the samples. SEM showed well-developed pores on the surface of the precursors after acid treatment, FTIR spectra revealed reduction, broadening, disappearance or appearance of new peaks after acid activation. EDX results showed highest percentage of carbon by atom respectively in the order BHA > KNPA > CHA respectively. The pHpzc was found to be 5.32, 4.57 and 3.69 for KNPA, BHA and CHA, respectively. Boehm titration result compliments that of pHpzc, indicating that the surfaces of the prepared adsorbents are predominantly acidic. This study promotes a sustainable innovative use of agro-wastes in the production of cheap and readily available activated carbons, thereby ensuring more affordable water and effluent treatment adsorbents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is absolutely essential for sustenance of life, although millions of people worldwide are suffering from shortage of fresh and clean water. This is due to the rapid pace of industrialization, population expansion, and unplanned urbanization which have contributed largely to environmental pollution. Industries where textile, paper, rubber, plastics, paints and leather are manufactured discharge colored effluents into the environment, metals come from different sources like mining, metal cleaning, plating baths, pulp, paper and paper board mills, refineries and fertilizer industries (Tan et al. 2008; Jordi et al. 2009). The effluents discharged from these industries into water bodies have raised much concern because of potential health hazards associated with the entry of these toxic components into the food chain (Helen and Lima 2010). The presence of dyes, pigment and heavy metals in effluents from industries are some of the factors that lowers water quality, these pollutants are potential cause of adverse effects on health, increased environmental toxicity and poor aesthetic quality of water depending on the concentration of these pollutants in the environment (Jesus et al. 2011; Jafar and Shajudha 2012; Yadav et al. 2013).
The removal of these pollutants from sewage and industrial waste water has become a major issue worldwide due to their possible toxic effects. The conventional methods that are employed for the removal of heavy metals, dyes and pigments are found to be inefficient and expensive, especially when treating wastewater with low concentration of heavy metals. Some of these methods generate chemical or biological sludge which cannot be recovered or regenerated (Seetha et al. 2012; Dauda et al. 2010; Rao et al. 2009; Aydin et al. 2008; El-Ashtoukhy et al. 2008; Sud et al. 2008; Dabrowski et al. 2004; Zhen et al. 2002; Volesky and Leusch 1995).
According to Ahmad and Hameed (2010); El-Ashtoukhy et al. 2008; Gong et al. (2008), various methods have been employed for waste water treatment, activated carbon adsorption was found to be superior for wastewater treatment compared to other physical and chemical techniques, as they possess inherent limitations such as high cost, formation of hazardous by-products and intensive energy requirements, activated carbon has emerged as a potential alternative to conventional physiochemical technologies in waste-treatment facilities. Adsorption is an effective separation process that has advantages in terms of cost, flexibility, simplicity of design, and ease of operation compared to other techniques. It does not result in the formation of harmful substances (Rafatullah et al. 2010).
Wastes generated from agricultural sector are many, they constitute nuisance to the immediate environment where they are found, agricultural wastes are usually in large quantity, some of these wastes are known for their offensive odor, their decayed matter have the ability to alter soil pH. Recently, different research works have been carried out, in other to prepare adsorbents from these agricultural wastes used as an alternative to commercially available activated carbon, which is expensive. Presently, agricultural waste materials have been proposed as economic and eco-friendly (Kumar and Kumar 2014). This will help in converting unwanted, surplus agricultural waste, of which billions of kilograms are produced annually, to value-added products (Vieira et al. 2009). These will protect the environment by getting rid of the danger that such waste can pose if left alone, most especially where disposal of agricultural wastes have become a major problem.
Adsorption process has proven to be one of the best water treatment technologies around the world, activated carbon is undoubtedly considered as a universal adsorbent for the removal of different types of pollutants from water (Ansari and Mohammad-Khah 2009; Bello et al. 2014). A number of agricultural waste materials have being studied for the removal of different pollutants from aqueous solutions at different operating conditions. They include: cassava peel (Horsfall et al. 2006), sugar beet pulp (Aksu and İşoğlu 2005), Nipha Palm (Wankasi et al. 2006), rice bran (Suzuki et al. 2007), coconut husk (Hameed et al, 2008; Tan et al, 2008), periwinkle shell (Bello et al. 2008), orange peel (Ningchuan et al. 2009), cocoa pod husk (Bello and Ahmad 2011), mango leaf (Khan et al. 2011), coconut shell (Bello and Ahmad 2012), loquat leaves (Akl and Salem 2012), durian seed (Bello et al. 2014), TiO2/UV, (Gupta et al. 2012), CNT/magnesium oxide composite (Saleh and Gupta 2012), fertilizer waste, (Gupta et al. 1998), waste material adsorbents (Mittal et al. 2011), waste material adsorbents (Mittal et al. 2009a), waste material adsorbents (Mittal et al. 2009b), waste material adsorbents (Gupta et al. 2010), tire derived carbons (Saleh and Gupta 2013), alumina-coated carbon nanotubes (Gupta et al. 2011), industrial wastes (Jain et al. 2003), Waste materials, (Mittal et al. 2010), Multi-walled carbon nanotubes–ionic liquid–carbon paste electrode (Khani et al. 2010), mesoporous activated carbon (Karthikeyan et al. 2012).
Activated carbons prepared from these precursors have been reported to be capable of removing metal ion and organic pollutant from aqueous solution. There are various types of dyes that are commonly used during dyeing processes such as basic dye, reactive dye, acidic dyes (Ahmad et al. 2014). These class of dyes differ in structural composition as well as in their interaction with adsorbent, the interaction is dictated by the nature of the adsorbent, such as morphology and the pH of the surface of adsorbent, this makes adsorbent to be effective towards adsorbate differently. Activated carbon materials are characterized by their large surface areas, high carbon content and better porosity which are well-developed. For these reasons, activated carbons are commercially used as adsorbents for the removal of organic chemicals and metal from the environment. Carbonaceous materials (animal, plant, or mineral origin) with high carbon content can be converted into activated carbon. This study examines the use of different agricultural waste materials as precursors for the preparation of cheap and readily available activated carbons.
Materials and methods
Preparation of the adsorbent
Sample collection and preparation
Kola nut pod, coconut husks were collected from dump sites while bean husk was obtained from Wazo market, Ogbomoso, Oyo State of Nigeria, where they were deposited. The samples were washed thoroughly with water to remove the dirt, the washed samples were then sun dried and pulverized.
Modification of the sample
Dried and pulverized samples were carbonized using muffle furnace, a carefully weighed 25.0 ± 0.01 g of raw sample were put into a beaker containing 500 cm3 of 0.3 M ortho-phosphoric acid (H3PO4). The content of the beakers were thoroughly mixed and heated on a hot plate until a thick paste was formed. The paste of each samples were then transferred into a evaporating dish which was placed in a furnace and heated at 300 °C for 30 min. Thereafter, the samples were allowed to cool and then washed with distilled water to a pH of 6.7 ± 0.12, oven dried at 105 °C for 4 h and the adsorbents were stored in an air-tight container for further use.
Characterization of adsorbents
Fourier transform infrared (FTIR)
Fourier transform infrared (FTIR) spectroscopic analysis was used to study the surface chemistry of raw and activated carbon prepared from the selected samples using FTIR (FTIR-2000, Perkin Elmer). The FTIR spectra gave information about the characteristics functional groups on the surface of the sample.
Scanning electron microscopy (SEM)
The scanning electron microscope (SEM) is one of the most versatile instruments available for the examination and analysis of the microstructure morphology. The scanning electron microscopy (SEM) is a technique based on electron–material interactions, capable of producing images of the sample surface. This technique was used to study the morphological feature and surface characteristics of the selected materials.
Energy dispersive X-ray (EDX)
Elemental analyses were carried out using EDX, it determines the component elements present in the samples. Line spectra (peaks) are obtained, each corresponding to a particular element. The intensity of the characteristic lines is proportional to the concentration of the element.
Determination of oxygen-containing functional groups
The Boehm titration method was used for this analysis. According to the method used by Ekpete and Horsfall (2011), 1.0 g of the raw and the activated carbons were kept in contact with 15 ml solution of NaHCO3 (0.1 M), Na2CO3 (0.05 M) and NaOH (0.1 M) for acidic group and 0.1 HCl for basic group/site, respectively, at room temperature for more than 2 days. Subsequently, the aqueous solution was back titrated with HCl (0.1 M) for acid and NaOH (0.1 M) for basic groups. The number and type of acidic site were calculated by considering that NaOH neutralizes carboxylic, lactonic and phenolic groups, Na2CO3 neutralizes carboxylic and lactonic groups and that NaHCO3 neutralizes only carboxylic groups. The amount of oxygen containing functional groups, F x , is calculated as follows:
where F x (mmolg−1) is the amount of oxygen-containing functional groups, V bx is the volume of titrant used to titrate the blank, V ex is the volume of the titrant used to titrate the extract, M t is the molarity of the titrant used and DF is the dilution factor.
Determination of point of zero charge (pHpzc)
The pH point of zero charge determination (pHpzc) of the raw and activated carbons of the samples were carried out by adding 0.1 g of activated carbon to 200 ml solution of 0.1 M NaCl whose initial pH has been measured and adjusted with NaOH or HCl. The containers were sealed and placed on a shaker for 24 h after which the pH was measured. The pHpzc occurs when there is no change in the pH after contact with adsorbent.
Results and discussion
Characterization of the prepared adsorbent
Fourier transform infrared spectral analysis
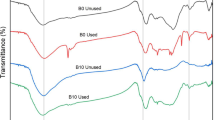
Figure 1 shows the FTIR spectra of kola nut pod, bean husk and coconut husk, respectively. The spectra of the samples show the presence of several functional groups. These spectra revealed a reduction, broadening, disappearance or appearance of new peaks after the process of acid activation. The shifts in the spectra revealed the effect of activation on these adsorbents. The prominent bands after activation are indications that the prepared adsorbent will be effective in the removal of dyes and heavy metals (Bello et al. 2015). The FTIR spectra of kola nut pod (Fig. 1a), shows a broad band at 3773 cm−1 representing the –OH stretching of alcohol or phenol. A sharp band observed at 3433–3428 cm−1 represents the –OH bonding group of alcohol or carboxyl group. The peak around 2382–2371 cm−1 corresponds to C≡N stretch vibration of amines. The band at 1856 cm−1 corresponds to C=O stretching of acid halide and the peak observed at 1790 cm−1 was assigned to C=O stretch of acid halide. The peak observed at 1640–1699 cm−1 represents C=C stretch of alkenes. The one at 1414–1472 cm−1corresponds to C–C stretching vibration of aromatic. The band observed at 1279–1254 cm−1 corresponds to C–H alkyl halide, 1107–1094 cm−1 were assigned to C–N stretch of aliphatic amines. The band at 679–696 cm−1 corresponds to C≡C bending of alkynes.
Also, Fig. 1b shows FTIR spectra of bean husk, a broad band observed at 3761 cm−1, represents bonded OH groups. The peak at 2930–2368 cm−1 shows an aliphatic C–H stretching vibration. 1757–1639 cm−1 shows C=O stretching of carboxylic group. The band observed at 1523 cm−1 corresponds to the secondary amine group. The peak shown at 1423 cm−1 indicates an aromatic C–C stretch and C–O stretch was observed at 1279 cm−1. The band around 1117 cm−1 shows presence of C=O bonds of ether, ester or phenols. O–H bending was observed at 633 cm−1.
Figure 1c is the FTIR spectra of coconut husk, the long bandwidth around 3433.29 cm−1 indicated that the main functional groups found on CHR and CHA; O–H stretching vibration of hydroxyl functional groups including hydrogen bonding. The band observed at 2926.01 cm−1 was assigned to the aliphatic C–H group. Other major peaks detected at bandwidths of 2376.30 cm−1, 1705–1739.79 cm−1, 1624.06–1618.28 cm−1 and 1377.17 cm−1 were, respectively, attributed to C≡C stretching of the alkyne group; vibration of carboxylic C=O stretching of lactones, ketones, and carboxylic anhydrides; C=C of aromatic ring; and C–H stretching in alkanes or alkyl group. The disappearance of ether and phenol in the CHA sample shows that these functional groups are thermally unstable (Ahmad et al. 2014).
The changes observed in the FTIR spectra of the selected sample confirm the effect of acid activation on the raw samples; this is an indication that these samples will be a useful adsorbent in waste water treatment (Bello et al. 2015).
Scanning electron micrograph (SEM)
Scanning electron micrograph (SEM) of kola nut pod raw (KNPR), kola nut pod activated (KHPA), bean husk raw (BHR), bean husk activated (BHA) and coconut husk raw (CHR), coconut husk activated (CHA) are shown in Fig. 2, respectively. The surface morphology of the selected samples clearly shows that KHPR, BHR and CHR surface pores were not properly developed, rough and uneven, and there are several pores formed, and are distributed over surface of the precursor after acid treatment. This is an indication that H3PO4 was effective in creating well-developed pores on the surface of the precursor, thus, leading to activated carbon with large surface area and porous surface structure. This was due to the breakdown of the lignocellulosic materials at high temperature followed by the evaporation of volatile compounds leaving samples with well-developed pores. The availability of pores and internal surface is requisite for effective adsorbent (Rao et al. 2006; Bello et al. 2015). Thus, the porous nature of the adsorbent prepared will help in adsorbent uptake which will be of help in adsorption process. These pores will provide a good surface for dye, heavy metals and other pollutant to be trapped and adsorbed onto (Bello et al. 2015; Ahmad et al. 2015a, b).
Energy dispersive X-ray
Elemental analyses of both raw and acid-activated samples were carried out using EDX to determine qualitatively and quantitatively the elements present in kola nut pod raw (KNPR), kola nut pod activated (KHPA), bean husk raw (BHR), bean husk activated (BHA) and coconut husk raw (CHR), coconut husk activated (CHA). The results obtained are shown in Table 1. Figure 3 shows the spectra of the results displayed in the Table 1. It was observed that carbon has the highest percentage by weight when compared with other elements present in the samples. Also carbon content increased after acid activation showing the effectiveness of the acid treatment on the precursors. According to Xiong and coworkers only samples with high carbon content can be an efficient adsorbent for the removal of dye, heavy metals and other organic pollutant from aqueous solution (Xiong et al. 2013). Effectiveness of the prepared adsorbent based on the carbon content in the removal of pollutant is in the order KNPA > BHA > CHA (Table 1). Results show that the most effective adsorbent among those prepared is KNPA followed by BHA and CHA.
Determination of pH point of zero charge
pH point of zero charge of activated carbon prepared from these waste materials were determined, the values were known by determining the point where the resulting curve cut through the pHo axis as shown in Fig. 4. The pHpzc was found to be 5.32, 4.57 and 3.69 mmol g−1 for KNPA, BHA and CHA, respectively. The combined influence of all the functional groups of activated carbon determines pHpzc, i.e., the pH at which the net surface charge on carbon was zero. At pH < pHpzc, the carbon surface has a net positive charge, while at pH > pHpzc the surface has a net negative charge (Al-Degs et al. 2000). Cation adsorption becomes enhanced at higher than the pHpzc, while adsorption of anions is equally enhanced at pH less than pHpzc (Almeidal et al. 2009; Farahani et al. 2011). This is consistent with the value of Boehm titration, showing the dominance of acidic groups on the surface of the prepared adsorbents (Table 2).
Determination of oxygen containing functional groups
Boehm titration technique was used to characterize the surface chemical property of the absorbent. Table 2 shows the Boehm titration result. The dominance of acidic groups on the activated carbon prepared from the selected material is higher than the basic groups, suggesting that the functional groups on the adsorbent surface are predominantly acidic. Higher acidic groups suggests more oxygenated functional groups, resulting in increase in adsorption capacity (Farahani et al. 2011).
Conclusion
This study revealed that the activated carbon prepared from selected agricultural wastes after acid activation and carbonization was effective in creating well-developed pores on the surface of the precursor, yielding product with high carbon content. Surface of the studied samples are acidic. These are indication that the activated carbon prepared from these selected agricultural wastes has promising ability, to remove different pollutants such as dye, metal ion and other organic pollutants. These adsorbents can be considered as suitable alternative precursors in lieu of the expensive commercial activated carbon. Moreover, since they are sourced locally, their sustained availability is guaranteed.
References
Ahmad AA, Hameed BH (2010) Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J Hazard Mater 175:298–303
Ahmad MA, Ahmad N, Bello OS (2014) Adsorptive removal of malachite green dye using durian seed-based activated carbon. Water Air Soil Pollut 225(8):1–18
Ahmad MA, Ahmad N, Bello OS (2015a) Removal of remazol brilliant blue reactive dye from aqueous solutions using watermelon rinds as adsorbent. J Disp Sci Technol 36(6):845–858
Ahmad MA, Afandi NS, Adegoke KA, Bello OS (2015b) Optimization and batch studies on adsorption of malachite green dye using rambutan seed activated carbon. Desalination Water Treat. doi:10.1080/19443994.2015.1119744
Akl MA, Salem NM (2012) Biosorption of copper(II) and lead(II) ions from aqueous solutions by modified loquat (Eriobotrya japonica) leaves (MLL). J Chem Eng Mater Sci 3(1):7–17
Aksu Z, İşoğlu A (2005) Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Proc Biochem 40:3031–3044
Al-Degs Y, Khraisheh M, Allen S, Ahmad M (2000) Effect of carbon surface chemistry on the reactive dyes from textile effluents. Water Res 34:927–935
Almeidal CAP, Debacher NA, Downs AJ, Cottet LC, Mello AD (2009) Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J Colloid Interface Sci 332:46–53
Ansari R, Mohammad-Khah A (2009) Activated charcoal: preparation, characterization and applications: a review article. Int J Chem Tech Res 1(4):859–864
Aydin H, Bulut Y, Yerlikaya C (2008) Removal of Cu(II) ion from aqueous solution by adsorption onto low-cost adsorbents. J Environ Manage 87(1):37–45
Bello OS, Ahmad MA (2011) Adsorptive removal of a synthetic textile dye using coca pod husks. Toxicol Environ Chem 93(7):1298–1308
Bello OS, Ahmad MA (2012) Coconut (Cocos nucifera) shell based activated carbon for the removal of malachite green dye from aqueous solutions. Sep Sci Technol 47(6):903–912
Bello OS, Adeogun AI, Ajaelu JC, Fehintola EO (2008) Adsorption of methylene blue onto activated carbon derived from periwinkle shells: kinetics and equilibrium studies. Chem Ecol 24(4):285–295
Bello OS, Adegoke KA, Bello OU, Lateef IO (2014) Sequestering Nikel (II) Ions from aqueous solution using various adsorbents: a review. Pak J Anal Environ 15(1):1–17
Bello OS, Adegoke KA, Akinyunni OO (2015) Preparation and characterization of a novel adsorbent from Moringa oleifera leaf. Appl Water Sci. doi:10.1007/s13201-015-0345-4
Dabrowski A, Hubicki Z, Podkoscielny P, Robens E (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56(2):91–106
Dauda BEN, Egila JN, Jimoh T (2010) Biosorptive removal of cobalt (II) ions from aqueous solution by Amaranthus hydridus L stalk wastes. Afr J Biotechnol 48(9):8192–8198
Ekpete OA, Horsfall MJ (2011) Preparation and characterization of activated carbon derived from fluted pumpkin stem waste (Telfairia occidentalis Hook F). Res J Chem Sci 1(3):10–17
El-Ashtoukhy ESZ, Amin NK, Abdelwahab O (2008) Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination 223(1):162–173
Farahani M, Abdullah S, Hosseini S, Shojaeipour S, Kasisaz M (2011) Adsorption-based cationic dyes using the carbon active sugarcane bagasse. Proc Environ Sci 10:203–208
Gong R, Zhu S, Zhang D, Chen J, Ni S, Guan R (2008) Adsorption behavior of cationic dyes on citric acid esterifying wheat straw: kinetic and thermodynamic profile. Desalination 230:220–228
Gupta VK, Srivastava SK, Mohan D, Sharma S (1998) Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions. Waste Manage 17(8):517–522
Gupta VK, Mittal A, Mittal J, Malviya A, Dipika Kaur D (2010) Decoloration treatment of a hazardous triarylmethane dye, Light Green SF (Yellowish) by waste material adsorbents. J Colloid Interf Sci 342(2):518–527
Gupta VK, Agarwal S, Saleh TA (2011) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazardous Mater 185(1):17–23
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng C 32(1):12–17
Hameed BH, Tan IAW, Ahmad AL (2008) Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem Eng 144(2):235–244
Helen KM, Lima RM (2010) Comparison of copper adsorption from aqueous solution using modified and unmodified hevea brasiliensis saw dust. Desalination 255:165–174
Horsfall MJ, Abia AA, Spiff AI (2006) Kinetic studies on the adsorption of Cd2+, Cu2+ and Zn2+ ions from aqueous solution by cassava (Manihot esculenta cranz) tuber bark waste. Bioresour Technol 97:283–291
Jafar AA, Shajudha BA (2012) Adsorption of copper from aqueous solution using low-cost adsorbent. Arch Appl Sci Res 4(3):1532–1539
Jain AK, Gupta VK, Bhatnagar A Suhas (2003) A comparative study of adsorbents prepared from industrial wastes for removal of dyes. Sep Sci Technol 38(2):463–481
Jesus AMD, Romão LPC, Araújo BR, Costa AS, Marques JJ (2011) Use of humin as an alternative material for adsorption/desorption of reactive dye. Desalination 274:13–21
Jordi L, Josep S, Joan L (2009) Modeling of the dynamic adsorption of an anionic dye through ion-exchange membrane adsorber. J Membr Sci 340:234–240
Karthikeyan S, Gupta VK, Boopathy R, Titus A, Sekaran G (2012) A new approach for the degradation of aniline by mesoporous activated carbon as a heterogeneous catalyst: kinetic and spectroscopic studies. J Mol Liquids 173(2012):153–163
Khan TA, Sharma S, Ali I (2011) Adsorption of rhodamine b dye from aqueous solution onto acid activated mango (Magnifera indica) leaf powder: equilibrium, kinetic and thermodynamic studies. J Toxicol Environ Health Sci 3(10):286–297
Khani H, Rofouei MK, Arab P, Gupta VK, Vafaei Z (2010) Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion (II). J Hazard Mater 183:402–409
Kumar B, Kumar U (2014) Removal of malachite green and crystal violet dyes from aqueous solution with bio-materials: a review. Glo J Res Eng 14(4):51–60
Mittal A, Kaur D, Malviya A, Mittal J, Gupta VK (2009a) Adsorption studies on the removal of coloring agent phenol red from wastewater using waste materials as adsorbents. J Colloid Interf Sci 337(2):345–354
Mittal A, Mittal J, Malviya A, Gupta VK (2009b) Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J Colloid Interf Sci 340(1):16–26
Mittal A, Mittal J, Malviya A, Gupta VK (2010) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interf Sci 344(2):497–507
Ningchuan F, Xueyi G, Sha L (2009) Adsorption study of copper (II) by chemically modified orange peel. J Hazard Mater 164(2):1286–1292
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177(1):70–80
Rao MM, Ramesh A, Purna CR, Seshaiah K (2006) Removal of copper and cadmium from the aqueous solution by activated carbon derived from Ceiba pentandra hulls. J Hazard Mater 129:123–129
Rao MM, Reddy DH, Venkateswarlu P, Seshaiah K (2009) Removal of mercury from aqueoususing activated carbon prepared from agricultural by-product/waste. J Environ Manage 90(1):634–643
Saleh TA, Gupta VK (2012) Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ Sci Pollut Res 19:1224–1228
Saleh TA, Gupta VK (2013) Processing methods, characteristics and adsorption behavior of tire derived carbons: a review Adv. Colloid Interf Sci 211:93–101
Seetha RR, Nageswara RV, Rajendra PP, Chitti BN (2012) Sorption of lead (II) Ion from wastewater using carica papaya leaf powder. Int J Eng Sci Adv Technol 2(6):1577–1581
Sud D, Garima M, Kumar MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions–a review. Bioresour Technol 99(14):6017–6027
Suzuki RM, Andrade AD, Sousa JC, Rollemberg MC (2007) Preparation and characterization of activated carbon from rice bran. Bioresour Technol 98(10):1986–1991
Tan IA, Ahmad AL, Hameed BH (2008) Adsorption of basic dye on high-surface -area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies. J Hazard Mater 154:337–347
Vieira AP, Santana SAA, Bezerra CWB, Silva HAS, Chaves JAP, Melo JCP, Filho Edson CS, Airoldi C (2009) Kinetics and thermodynamics of textile dye adsorption from aqueous solutions using babassu coconut mesocarp. J Hazard Mater 166:1272–1278
Volesky B, Leusch A (1995) The influence of film diffusion on cadmium biosorption by marine biomass. J Biotechnol 43:1–10
Wankasi D, Horsfall MJ, Spiff AI (2006) Sorption kinectics of Pb(II) and Cu(II) ion from aqueous solution by Nipah Palm (Nypafrutie answurmb) shoot biomass. Electron J Biotechnol 9:1–7
Xiong C, Jila Q, Chen X, Wang G, Yao C (2013) Optimization of polyacrylonitrile-2-aminothiazole resin synthesis, characterization, and its adsorption performance and mechanism for removal of Hg(II) from aqueous solution. Ind Eng Chem Res 52:4978–4986
Yadav SK, Sinha S, Singh DK (2013) Removal of Lead (II) from aqueous solution using papaya seed carbon: characteristics and kinetics study. Int J Civil Environ Eng 4(2):127–136
Zhen GS, Xian DL, Chun CW, Huai MC, Hong C (2002) Lead, phyto extraction from contaminated soil with high biomass plant species. J Environ Qual 31(6):1893–1900
Acknowledgements
The corresponding author acknowledges the support obtained from The World Academy of Science (TWAS) in form of Grants; Research Grant Numbers: 11-249 RG/CHE/AF/AC_1_ UNESCO FR: 3240262674 (2012) and 15-181 RG/CHE/AF/AC_1_: 3240287083 (2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bello, O.S., Owojuyigbe, E.S., Babatunde, M.A. et al. Sustainable conversion of agro-wastes into useful adsorbents. Appl Water Sci 7, 3561–3571 (2017). https://doi.org/10.1007/s13201-016-0494-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0494-0