Abstract
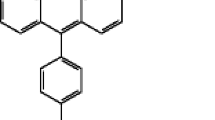
Chemically prepared activated carbon derived from durian seed (DSAC) was used as adsorbent to adsorb Malachite green (MG) dye. The prepared DSAC was characterized using Brunauer–Emmet–Teller (BET), Fourier transform infrared (FTIR), scanning electron microscope (SEM), and proximate analysis, respectively. Batch adsorption studies were carried out for the removal of MG dye from aqueous solutions by varying operational parameters like contact time, initial MG dye concentration, solution temperature, and initial solution pH. Maximum dye removal of 97 % was obtained at pH 8. Experimental data were analyzed by eight model equations—Langmuir, Freundlich, Temkin, Dubinin–Radushkevich, Radke–Prausnitz, Sips, Vieth–Sladek, and Brouers–Sotolongo isotherms—and it was found that the Freundlich isotherm model fitted the adsorption data the most. Adsorption rate constants were determined using pseudo-first-order and pseudo-second-order rate equations, Elovich, intraparticle diffusion, and Avrami kinetic model. The results clearly showed that the adsorption of MG dye onto DSAC followed the pseudo-second-order model, and the mechanism of adsorption was controlled both by film diffusion and intraparticle diffusion. Thermodynamic parameters such as ∆G, ∆H, and ∆S were also calculated for the adsorption process. The process was found to be spontaneous and endothermic in nature. This work provided an attractive adsorbent for the removal of MG dye from wastewaters.










Similar content being viewed by others
References
Aharoni, C., & Ungarish, M. (1976). Kinetics of activated chemisorptions. Part I: the non-Elovichian part of the isotherm. Journal of Chemical Society, Faraday transaction, 72, 265–268.
Ahmad, M. A., & Alrozi, R. (2010). Optimization of preparation conditions for mangosteen peel-based activated carbons for the removal of Remazol Brilliant Blue R using response surface methodology. Chemical Engineering Journal, 165, 883–890.
Ahmad, M. A., & Alrozi, R. (2011). Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: equilibrium, kinetic and thermodynamic studies. Chemical Engineering Journal, 171, 510–516.
Ahmad, R., & Kumar, R. (2010). Adsorption studies of hazardous malachite green onto treated ginger waste. Journal of Environmental Management, 91, 1032–1038.
Ahmad, A. A., Hameed, B. H., & Ahmad, A. L. (2009). Removal of disperses dye from aqueous solution using waste-derived activated carbon: optimization study. Journal of Hazardous Materials, 170, 612–619.
Alkan, M., Dogan, M., Turhan, Y., Demirbas, O., & Turan, P. (2008). Adsorption kinetics and mechanism of maxilon blue 5G dye on sepiolite from aqueous solution. Chemical Engineering Journal, 139, 213–223.
Arivoli, S., Hema, M., Martin, P., & Prasath, D. (2009). Adsorption of malachite green onto carbon prepared from borassus bark. Arabian Journal of Science and Engineering, 34, 31–36.
Auta, M., & Hameed, B. H. (2011). Optimized waste tea activated carbon for adsorption of Methylene Blue and Acid Blue 29 dyes using response surface methodology. Chemical Engineering Journal, 175, 233–243.
Avrami, M. (1940). Kinetics of phase change: transformation-time relations for random distribution of nuclei. Journal of Chemical Physics, 8, 212–224.
Baskaralingam, P., Pulikesi, M., Elango, D., Ramamurthi, V., & Sivanesan, S. (2006). Adsorption of acid dye onto organobentonite. Journal of Hazardous Materials 128(2–3), 138–144.
Basta, A. H., Fierro, V., El-Saeid, H., & Celzard, A. (2009). 2-Steps KOH activation of the rice straw, an efficient method for preparing high performance activated carbons. Bioresources Technology, 100, 3941–3947.
Bekci, Z., Cavas, Y., & Seki, L. (2009). Removal of malachite green by using an invasive marine alga Caulerpa racemosa var. cylindracea. Journal of Hazardous Materials, 161, 1454–1460.
Bello, O. S., & Ahmad, M. A. (2012). Coconut (Cocos nucifera) shell based activated carbon for the removal of malachite green dye from aqueous solutions. Separation Science Technology, 47(6), 903–912.
Bello, O. S., Adeogun, A. I., Ajaelu, J. C., & Fehintola, E. O. (2008). Adsorption of methylene blue onto activated carbon derived from periwinkle shells: kinetics and equilibrium studies. Chemistry and Ecology, 4(24), 285–295.
Bello, O. S., Ahmad, M. A., & Ahmad, N. (2011a). Adsorptive features of banana (Musa paradisiaca) stalk-based activated carbon for malachite green dye removal. Chemistry and Ecology, 28, 153–167.
Bello, O. S., Siang, T. T., & Ahmad, M. A. (2011b). Adsorption of remazol brilliant violet 5R reactive dye from aqueous solution by cocoa pod husk-based activated carbon: kinetic, equilibrium and thermodynamic studies. Asia Pacific Journal of Chemical, 7, 378–388.
Boehm, H. P. (2002). Surface oxides on carbon and their analysis: a critical assessment. Carbon, 40, 145–149.
Booncherm, P., & Siriphanich, J. (1991). Postharvest physiology of durian pulp and husk. Kasetsart Journal, 25, 119–125.
Boyd, G. E., Adamson, A. W., & Myers, L. S. (1947). The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics Journal of the America Chemistry Society, 69, 2836–2848.
Brouers, F., Sotolongo, O., Marquez, F., & Pirard, J. P. (2005). Microporous and heterogeneous surface adsorption isotherms. Physica A: Statistical Mechanics and its Applications, 349, 271–282.
Chu, K. H. (2002). Removal of copper from aqueous solution by chitosan in prawn shell: adsorption equilibrium and kinetics. Journal of Hazardous Material, 90, 77–95.
Djeribi, R., & Hamdaoui, O. (2008). Sorption of copper(II) from aqueous solutions by cedar sawdust and crushed brick. Desalination, 225, 95–112.
Dogan, M., Abak, H., & Alkan, M. (2009). Adsorption of methylene blue onto hazelnut shell: kinetics, mechanism and activation parameters. Journal of Hazardous Material, 164, 172–181.
Dubinin, M. M., Radushkevich, L. V., (1947). Equation of the characteristic curve of activated charcoal. Proceedings of Sciences and Physical Chemical Section USSR 55,331.
Freundlich, H. M. F. (1906). Over the adsorption in solution. The Journal of Physical Chemistry, 57, 385–471.
Godbole, P. T., & Sawant, A. D. (2006). Removal of malachite green from aqueous solutions using immobilized Saccharomyces cerevisiae. Journal of Scientific and Industrial Research, 65, 440–442.
Guechi, E.-K., & Hamdaoui, O. (2011). Sorption of malachite green from aqueous solution by potato peel: kinetics and equilibrium modeling using non-linear analysis method. Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2011.05.011.
Gupta, N., Kushwaha, A. K., & Chattopadhyaya, M. C. (2011). Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2011.07.021.
Hameed, B. H., Ahmad, A. A., & Aziz, N. (2007). Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chemical Engineering Journal, 133, 195–203.
Ho, Y. S., McKay, G. (1999) Pseudo-second order model for sorption processes. Process Biochemistry 34, 451–465.
IUPAC, IUPAC manual of symbols and terminology, Pure Appl. Chem. 31 (1972)587.http://old.iupac.org/reports/2001/colloid_2001/manual_of_s_and_t/node15.html.
Khan, A. A., Ahmad, R., Khan, A., & Mondal, P. K. (2010). Preparation of unsaturated polyester Ce (IV) phosphate by plastic waste bottles and its application for removal of malachite green dye from water samples. Arabian Journal Chemistry. doi:10.1016/j.arabjc.2010. 10.012.
Khattri, S. D., & Singh, M. K. (2009). Removal of malachite green from dye wastewater using neem sawdust by adsorption. Journal Hazardous Materials, 167, 1089–1094.
Kilic, M., Apaydin-Varol, E., & Pütün, A. E. (2011). Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: equilibrium, kinetics and thermodynamics. Journal of Hazardous Materials, 189, 397–403.
Koprivanac, N., & Kusic, H. (2008). Hazardous organic pollutants in colored wastewaters. New York: Nova Science Publishers.
Kumar, K. V., & Sivanesan, S. (2007). Isotherms for malachite green onto rubber wood (Hevea brasiliensis) sawdust: comparison of linear and non-linear methods. Dyes and Pigments, 72, 124–129.
Lagergren, S. (1898). Zur Theorie der sogenannten Adsorption Geloester Stoffe. Veternskapsakad Handlingar, 24, 1.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemistry Society, 40, 1361.
Li, K., Zheng, Z., Huang, X., Zhao, G., Feng, J., & Zhang, J. (2009). Equilibrium, kinetic and thermodynamic studies on the adsorption of 2-nitroaniline onto activated carbon prepared from cotton stalk fibre. Journal of Hazardous Materials, 166, 213–220.
Mahmoodi, N. M., Hayati, B., Arami, M., & Lan, C. (2011). Adsorption of textile dyes on pine cone from colored wastewater: kinetic, equilibrium and thermodynamic studies. Desalination, 268, 117–125.
Mahmoud, D. K., Mohd Salleh, M. A., Wan Abdul Karim, W. A., Idris, A., & Zainal Abidin, Z. (2012). Batch adsorption of basic dye using acid treated kenaf fibre char: equilibrium, kinetic and thermodynamic studies. Chemical Engineering Journal, 181–182, 449–457.
Mall, D., Srivastava, V. C., Agarwal, N. K., & Mishra, I. M. (2005). Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon—kinetic study and equilibrium isotherm analyses. Colloids Surface, A 264, 17–28.
Nethaji, S., Sivasamya, A., Thennarasua, G., & Saravanan, S. (2010). Adsorption of Malachite Green dye onto activated carbon derived from Borassus aethiopum flower biomass. Journal of Hazardous Materials, 181, 271–280.
Nowicki, P., Pietrzak, H., & Wachowska, R. (2010). Active carbons prepared by chemical activation of plum stones and their application in removal of NO2. Journal of Hazardous Material, 181, 1088–1094.
Olivares, M. M., Fernandez-Gonzalez, C., Macías-García, A., & Gómez-Serrano, V. (2006). Preparation of activated carbon from cherry stones by chemical activation with ZnCl2. Applied Surface Science, 252, 5967–5971.
Parimaladevi, P., & Venkateswaran, V. (2011). Kinetics, thermodynamics and isotherm modeling of adsorption of triphenylmethane dyes (methyl violet, malachite green and magenta II) onto fruit waste. Journal of Applied Technology in Environmental Sanitation, 1, 273–283.
Parthasarathy, S., Manju, N., Hema, M., & Arivoli, S. (2011). Removal of malachite green from industrial waste-water by activated carbon prepared from cashew nut bark Alfa Universal. International Journal of Chemistry, 2, 41–46.
Radke, C. J., & Prausnitz, J. M. (1972). Industrial engineering chemical fundamental, 11, 445–451.
Saha, P., Chowdhury, S., Gupta, S., Kumar, I., & Kumar, R. (2010). Assessment on the removal of malachite green using tamarind fruit shell as biosorbent. Clean Soil Air Water, 38, 437–445.
Santhi, T., Manonmani, S., & Smitha, T. (2010a). Removal of malachite green from aqueous solution by activated carbon prepared from the epicarp of Ricinus communis by adsorption. Journal of Hazardous Materials, 179, 178–186.
Santhi, T., Manonmani, S., & Smitha, T. (2010b). Kinetics and isotherm studies on cationic dyes adsorption onto Annona squamosa seed activated carbon. International Journal of Engineering Science and Technology, 2, 287–295.
Santhi, T., Manonmani, S., Vasantha, V. S., & Chang, Y. T. (2011). A new alternative adsorbent for the removal of cationic dyes from aqueous solution. Arabian Journal of Chemistry. doi:10.1016/ j.arabjc.2011.06.004.
Sartape, A. S., Mandhare, A. M., Jadhav, V. V., Raut, P. D., Anuse, M. A., & Kolekar, S. S. (2013). Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2013.12.019.
Sekhar, C. P., Kalidhasan, S., Rajesh, V., & Rajesh, N. (2009). Biopolymer adsorbent for the removal of malachite green from aqueous solution. Chemosphere, 77, 842–847.
Sips, R. (1948). Combined form of Langmuir and Freundlich equations. Journal of Chemistry and Physics, 16, 490–495.
Sonawane, G.H. Shrivastava, V.S. (2009). Kinetics of decolourization of malachite green from aqueous medium by maize cob (Zea maize): an agricultural solid waste. Desalination 247, 430–441.
Syed, S. P. S. (2011). Study of the removal of malachite green from aqueous solution by using solid agricultural waste. Research Journal of Chemical Science, 1, 88–93.
Tan, I.A.W., (2008). Preparation, characterization and evaluation of activated carbons derived from agricultural by product desorption of methylene blue and 2,4,6-trichlorophenol, PhD. Thesis, Universiti Sains Malaysia.
Temkin, M. I., & Pyzhev, V. (1940). Kinetics of ammonia synthesis on promoted iron catalyst. Acta Physiochimica USSR, 12, 327–356.
Tsai, W. T., & Chen, H. R. (2010). Removal of malachite green from aqueous solution using low-cost Chlorella-based biomass. Journal of Hazardous Material, 175, 844–849.
Tunç, Ö., Tanac, H., & Aksu, Z. (2009). Potential use of cotton plant wastes for the removal of Remazol Black B reactive dye. Journal of Hazardous Materials, 163, 187–198.
Vieth, W. R., & Sladek, K. J. (1965) A model for diffusion in a glassy polymer. Journal of Colloid Science 20, 1014–1033.
Wang, L., Zhang, J., Zhao, R., Li, C., Li, Y., & Zhang, C. (2010). Adsorption of basic dyes on activated carbon prepared from Polygonum orientale Linn: equilibrium, kinetic and thermodynamic studies. Desalination, 254, 68–74.
Wang, S., Boyjoo, Y., Choueib, A., & Zhu, Z.H. (2005). Removal of dyes from aqueous solution using fly ash and red mud. Water Research 39, 129–138.
Weber, W. J., & Morris, J. C. (1962). Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division ASCE, 89, 31–59.
Wu, F. C., Tseng, R. I., & Jung, R. S. (2001) Kinetic modelling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Research 35, 613–618.
Zhang, J., Li, Y., Zhang, C., & Jing, Y. (2008). Adsorption of malachite green from aqueous solution onto carbon prepared from Arundo donax root. Journal of Hazardous Materials, 150, 774–782.
Acknowledgments
The financial support in the form of grants from USM; the 3 months USM-TWAS Visiting Researcher Fellowship, FR number: 3240268492 awarded to the corresponding author; and the accumulated leave granted to Dr. O.S Bello by his home institution to utilize the fellowship is thankfully recognized.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, M.A., Ahmad, N. & Bello, O.S. Adsorptive Removal of Malachite Green Dye Using Durian Seed-Based Activated Carbon. Water Air Soil Pollut 225, 2057 (2014). https://doi.org/10.1007/s11270-014-2057-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2057-z