Abstract
Surface water samples from three stations in the Bonny/New Calabar River Estuary were analyzed for the physicochemical characteristics and trace metal level in 2011 and 2012, respectively. Results show pH ranged from 7.56 to 7.88 mg/L; conductivity, 33,489.00 to 33,592.00 µScm−1; salinity, 15.33 to 15.50 ‰; turbidity, 4.35 to 6.65 NTU; total dissolved solids, 22111.00 to 23263.00 gm−3; dissolved oxygen, 4.53 to 6.65 mg/L; and biochemical oxygen demand, 1.72 mg/L. The level of some trace metals (Ca, Mg, K, Zn, Pb, Cd, Co, Cr, Cu, Fe, Ni, and Na) were also analyzed by Atomic absorption spectrometry with K, Zn, and Co being statistically significant (P < 0.05). The results were compared with USEPA and WHO Permissible Limits for water quality standards. It was observed that the water quality parameters in the Bonny Estuary show seasonal variation with higher values for pH, DO, BOD, temperature, and salinity during the dry season than wet season. Concentrations of trace metals such as Pb, Cd, Zn, Ni, and Cr were higher than stipulated limits by WHO (2006). The result of the Metal Pollution Index suggests that the river was slightly affected and therefore continuous monitoring is necessary to avert possible public health implications of these metals on consumers of water and seafood from the study area.
Similar content being viewed by others
Introduction
The coastal areas of Nigeria, particularly the Niger Delta Basin, have suffered debilitating environmental degradation and pollution from human activities such as oil industry operations, manufacturing, and municipal discharges. Urbanization and municipal activities have also contributed to the amount of wastes which includes solid, liquid, gaseous emissions, and heavy metals deposited on the environment which may result in the contamination of our environment (Onojake and Frank 2013).
Water quality monitoring has become a subject of concern in marine, stream, and river water due uncontrolled disposal of urban effluents, runoff, atmospheric deposition, municipal, and industrial effluent into these water bodies (Onojake et al. 2011).
In Africa and developing world where environmental protection laws have not been enforced, industrial and domestic wastes are dumped indiscriminately into water bodies. These wastes have been reported to contain toxic and hazardous substances including heavy metals, which eventually settle in bottom sediments (Oguzie 2002).
Several workers have investigated the concentration of heavy metals in the coastal rivers with Atlantic tidal effect in Nigeria. A good account of heavy metal concentrations in the Niger Delta is given in the work of Asonye et al. (2007). Trace metals are among the most common environmental pollutants and their occurrence in rivers and biota indicates the presence of natural or anthropogenic source (Mohiuddin et al. 2001). The existence of trace metals in aquatic environments has led to serious concerns about their influence on plant and animal life (Sheikh et al. 2007; Zvinowanda et al. 2009).
Industrial wastes have the potential to contribute strong acid to a water body and may cause dented effects on both the fauna and flora. Disposal of waste material into water bodies as well as effluents from industries and urbanization in developing countries is some activities which can probably introduce trace metals and eventually led to deterioration of the water quality in recent years. These activities lead to the generation of municipal and industrial wastes which pose problems of disposal and sometimes, are quickly disposed into the nearby water bodies (Akaninwor et al. 2007).
The Bonny/New Calabar River Estuary, which is the site of choice, has been the focal point of multidisciplinary research because there have been substantial increases in the industrial and agricultural development over the past four decades with attendant population growth. Apparently, these activities have resulted in the direct discharge of organic and inorganic substances including crude oil and refined products through normal operation (as effluents), operational failures, and sabotage to facilities into the adjoining water bodies.
This study was designed to investigate the physicochemical properties and the level of some trace metals in Bonny/New Calabar River Estuary, Niger Delta, Nigeria.
Materials and methods
Description of study site
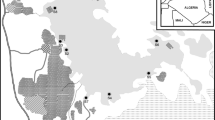
The sampling stations are shown in Fig. 1. A total of three stations were sited at 1000 m interval along the Bonny River.
Station 1 (4°45′04.03″ and 7°00′14.08″) was located downstream of the Bonny River system. The sampled point was between the Nembe waterside which is a jetty for local transport and journeys outside Port Harcourt using diesel and petro engine boats and the dock compound of the Ibeto cement bagging factory. In addition, it serves as an area for fishing and human settlements. Nembe waterside witnesses a lot of activities as a result of a large number of people that patronize a popular market; the Creek road market, located behind the jetty, where a lot of buying and selling is done for food and other products.
Nembe waterside Port Harcourt is subject to human-induced pressures resulting from urbanization, industrialization, and intensive navigation. It links Port Harcourt city with Bonny Island where most of the oil installations in Rivers State are situated. It also links the Island directly with the Atlantic ocean through which crude oil is exported by massive oil tankers. Previous researches shows that the Seasonal variation of rainfall (mm) in the Niger Delta during the wet season (March to November) is from 2301 to 3670 mm and during the dry season (December to March) is 43 to 97 mm (Adejuwon 2012).
Stations 2 and 3 (4°44′12.21″ and 4°46′33.73″ and 7°00′01.15″ and 7°00′18.85″) were located upstream of the Bonny River system. Sampled points were at the wharf of the Nigerian Ports Authority and at a point close to the oil terminal. Both areas receive refinery effluent which passes through the dug-in channels from petroleum tank farm to the discharge points, which is the Bonny River. Both areas are of brackish water type and are central points for development and commercial activity but have the reputation of being the most polluted. Sources of pollution for both locations include organic waste, litter, petroleum hydrocarbons, and toxic chemicals.
The Niger Delta lies mainly within the wet equatorial climatic which experience long-rainy season from March to November, with mean annual rainfall for the region under study for the past 29 years (1984–2014) ranges from 1500 mm around the northern fringe to 4500 mm around the coastal margin (Adejuwon 2012; Igweze et al. 2014).
Province geology of Niger Delta
The study area lies within the Niger Delta; its geology is therefore typical of the Niger Delta Basin. The area forms part of a geological sequence of the Quaternary and Tertiary formations of the Niger Delta, consisting mainly of three main geologic formations: The Benin Formation, Agbada Formation, and Akata Formation (Sundararaman et al. 2002). It is situated in the Gulf of Guinea and extends throughout the Niger Delta Province, as defined by Klett et al. (1997). From the Eocene to the present, the delta has prograded southwestward, forming depobelts that represent the most active portion of the delta at each stage of its development (Doust and Omatsola 1990). These depobelts form one of the largest regressive deltas in the world with an area of some 300,000 km2 (Kulke 1995), a sediment volume of 500,000 km3 (Hospers 1965) and a sediment thickness of over 10 km in the basin depocenter (Kaplan et al. 1994). There are the 2-km sediment thickness contours or 4000-m bathymetric contour, in areas where sediment thickness is >2 km, to the south and southwest (Michele et al. 1999). The quaternary deposits are considered universally to be the recent expressions of and continuation of the Benin Formation and consist of medium to coarse-grained sands, sandy clays, silts and subordinate, lensoid clay bands thought to have been deposited during quaternary interglacial marine transgressions (Durotoye 1989). Amajor (1991) has shown that they are an admixture of fluvial/tidal channel, tidal flats, and mangrove swamp deposits. The sands are micaceous and feldspathic, subrounded to angular in texture and constitute good aquifers.
The Benin Formation is essentially fluvial in origin comprising unconsolidated, massive, and porous fresh water bearing sands with localized clay/shale beds. The thickness of the Benin Formation is about 2100 m on the average. The age of this Formation ranges from Miocene to Recent. The Agbada Formation underlies the Benin Formation. Its thickness varies from 300 to 4500 m (Short and Stauble 1967). This formation consists predominantly of unconsolidated pebbles and very coarse to fine-grained sand units with subordinate shale beds. Underneath the Agbada Formation is the Akata Formation of Eocene to recent age (Amadi et al. 1987), which is made up of shales with local inter-bedding of sands and siltstones. It was deposited in a typically marine environment with maximum thickness of about 6100 m (Ofoegbu1989).
Sample collection and analysis
Surface water samples were collected at a depth of 0–100 cm, quarterly over a 2-year period (2011–2012), with 1 L plastic containers that were pre-rinsed with trioxonitrate (v) acid for 24 h and rinsed with de-ionized water. The samples were then placed in a box containing ice packs, transported to the Laboratory, and stored at a temperature of about 4 °C prior to analyses. The samples were analyzed quantitatively in the laboratory for the following physicochemical parameters such as pH, temperature, conductivity, salinity, turbidity, dissolved oxygen (DO), biochemical oxygen demand (BOD), total dissolved solids (TDS), and some trace metals according to standard methods (American Public Health Association 1995).
All samples were run in triplicates and the relative standard deviation for the triplicate analysis was less than 10 %. Standard solutions of the metals were prepared from their 1000 ppm stock solutions for calibration. The concentrations of the metals (Ca, Mg, K, Zn, Pb, Cd, Co, Cr, Cu, Fe, Ni, and Na) were determined using a varian atomic absorption spectrophotometer (spectra AA-100).
Metal Pollution Index (MPI)
The Metal Pollution index (MPI) is one of the indices for determining the quality of water. It is a method of rating that shows the composite influence of individual parameters on the overall quality of water (Tamasi and Cini 2004). The rating is a value between zero and one, reflecting the relative importance individual quality considerations. Amadi (2012) has shown that the higher the concentration of a metal compared to its maximum allowable limit, the worse the quality of the water. Metal pollution Index application can be for quality of sea, river water as well as drinking water (Filatov et al. 2005; Lyulko et al. 2001; Amadi 2012).
To compare the total metal content at the different sampling sites, the metal pollution index (MPI) was used according to the equation proposed by Usero et al. (1997).
where Cf n concentration of the metal n in the sample.
MPI value >1 is a threshold of warning.
Results and discussion
The results of the physicochemical characteristics and trace metal concentration of the Bonny/New Calabar Estuary for the three stations are shown in Tables 1, 2 and 3.
pH
pH is a measure of the free hydrogen ion and hydroxyl ions in the water. pH is an important indicator of water that is changing chemically. The observed values were generally characteristic of tidal brackish water environment as noted by Ajao and Fagade (2002). In addition, the values obtained were within the permissible range of values (6.5–8.5) prescribed as standard for water quality (Table 1). This confirms that the water is suitable for aquatic life (Zhou et al. 1999).
Furthermore, the seasonal variation observed was in agreement with results of pervious study conducted by Dublin-Green (1990) in the Bonny Estuary with the highest values recorded during the dry season and lowest values during wet season. This could be linked to increase photosynthetic rates of the aquatic plants (Ekeh and Sikoki 2003; Ansa 2005).
Temperature
The highest temperature was recorded during the dry season compared with the wet season. This observation was in agreement with the findings earlier reported in the Niger Delta waters, as reported by (Sikoki and Zabbey 2006; and Jamabo 2008). On the other hand, the drop in temperature of surface waters in the wet season is attributable to heavy rainfall as the region have high quantum of rainfall and also long period of rainy season. The aquatic organisms are, however, adapted to such changes in temperature and can even withstand changes outside this range.
Conductivity
Conductivity is a measure of the conductance of an electric current in water. It relates closely to the total dissolved solids (mineral) content of water. The higher conductivity observed in the wet season could be due to large volumes of water from the sea. It was also observed that stations 1 and 3 were significantly different from station 2, while stations 1 and 3 were not significantly different.
Salinity
The observed values of salinity as seen in Table 1 might be attributed to the proximity of the Bonny River to the sea. Seasonal variation displayed higher values during the dry season than wet season. This could be attributable to high sunlight intensity which increased water evaporation rate of surface water making it saltier and more saline. In addition, the months of April to October in West Africa usually coincide with the rainy season when high volumes of freshwater are discharged into coastal or estuarine waters that lower or dilute the water. According to Mclusky (1989), it was reported that rainfall could cause dilution of estuarine water hence reduction in saline.
Dissolved oxygen (DO)
The recorded values DO were observed to be higher during the dry season than wet season. The lowered DO during the wet season might be explained by the reduced photoperiod and the photosynthetic activities of aquatic plants. However, the range of DO was still within the acceptable limit for aquatic life. Dissolved oxygen in water can be depleted as it is used in the oxidation of organic matter thereby reducing its magnitude in water. DO also may be consumed by the bioxidation of nitrogenous material in water (Onojake et al. 2011).
Biological oxygen demand
The values of BOD obtained during the wet and dry seasons were <10 mg/L stipulated by USEPA, implying that the water body was fairly clean. The slightly higher value obtained in station 1, could be attributable to the influence of the municipal activities going on within the area. The higher BOD in the dry season (2.83 mg/L) could be the effect of higher temperature, salinity, and putrefaction of substances deposited in the river.
Total dissolved solid (TDS)
The observed values of total dissolved solids (TDS) were higher than the recommended value for brackish water (Mcneely et al. 1979). This is indicative of organic pollution from anthropogenic sources, such as domestic sewage, septic tanks, and agricultural activities. (Saad et al. 1994). The higher TDS concentration in the wet season (26,400.00 gm−3) might be due to high surface runoffs, overland flow, and higher discharge of organic waste into the river. High concentrations of TDS may affect taste adversely and deteriorate plumbing and appliances. The USEPA recommends that water containing more than 2000 mg/L of dissolved solids not be used if other less mineralized supplies are available.
Turbidity
Turbidity is a measure of suspended minerals, bacteria, plankton, and dissolved organic and inorganic substances. Turbidity often is associated with surface water sources. The turbidity values were observed to be higher during the wet season than in the dry season. This was probably due to the high-natural erosion and runoffs from the surrounding as well as the waste generated from the municipal activities within the river.
Trace metals in water
Calcium and magnesium are the main contributors to water hardness. When water is heated, calcium breaks down and precipitates out of the solution, forming scale. The concentrations of calcium in the waters were well within stipulated limits of 5–500 mg/L by US EPA (2004) and WHO (2006). Concentrations of Mg, K, Fe, Ni, and Na were slightly higher in 2011 than 2012. Potassium concentrations in water are generally very small, but excessive amounts may have a laxative effect. The concentration of K varies between the 2 years at a statistical significant level of P < 0.05 (P = 0.0112) indicating increase in 2012 (Table 4). Zinc concentrations recorded a wider range in 2012 than 2011. The slight increase in the concentration was as a result of anthropogenic input. Furthermore, Zn values recorded in water samples during the two years showed levels higher than stipulated limits of 0.001 mg/L for drinkable water by WHO (2006). Elevated levels of Zn have been shown to adversely affect growth, survival, and reproduction animals (Eisler 1993).
The concentration of Pb was higher than stipulated limits of 0.01 mg/L for safe drinkable water by WHO (2006) in both 2011 and 2012. Adverse effects caused by Pb to aquatic organisms such as algae, benthic invertebrates, and embryos and fingerlings of freshwater fish and amphibians include loss of sodium, reduced capability, developmental problems, and distorted algal growth (Freda 1991). Cadmium values were higher than 0.003 mg/L stipulated by WHO (2006) as a safe limit in drinkable water. Cadmium is an important factor in aquatic monitoring studies, because it has been found to be toxic to fish and other aquatic organisms. It is one of the most toxic elements with widespread carcinogenic effects in humans, widely distributed in the aquatic environment, and bioaccumulates at all trophic levels, accumulating in the livers and kidneys of fish; Crustaceans appear to be more sensitive to cadmium than fish and mollusks (Sadiq 1992; Sindayigaya et al. 1994). Chromium values were higher in 2012 than 2011 and higher than stipulated limit of (0.05 mg/L) by WHO (2006). Chromium in its hexavalent form has been known as a carcinogen and implicated in many health effects in animals (WHO 2006). The concentration of copper was below permissible limits of 2 mg/L by WHO (2006) and US EPA (2004). The concentration of Fe was higher in 2011 than 2012. Nickel, cobalt, and sodium concentrations recorded in 2011 and 2012 were also below permissible limits stipulated by WHO (2006) and US EPA (2004) (Tables 1, 2). Although the results of surface water concentrations of heavy metals observed in the present study agrees with the general opinion of low-level heavy metal concentrations in the study area and Niger Delta (Asonye et al. 2007; Chindah et al. 2004; Ubalua et al. 2007a, b), some of the toxic metals such as Pb, Cd, Zn, Ni, and Cr were higher than stipulated limits by WHO (2006) and require continuous monitoring to detect malicious increases as a result of anthropogenic input and avert possible public health implications of these metals on consumers of water and seafood from the study area.
Metal Pollution Index
In determining the value of metal pollution index, the numbers of sampling points were increased to five, (Table 3). Some researchers have classified water quality using MPI. Class I is very pure with MPI less 0.3 (<0.3); class II is pure with MPI between 0.3 and 1.0; class III is slightly affected with MPI between 1.0 and 2.0; class IV is moderately affected with MPI between 2.0 and 4.0; class V is strongly affected with MPI 4.0–6.0; and class VI is seriously affected with MPI >6 (Lyulko et al. 2001; Caerio et al. 2005).
The concentrations of most of the metals in the Bonny/New Calabar River Estuary varied notably depending on the location of the sampling sites. Table 3 shows that metal pollution index (MPI) for all stations were between 1.0 and 2.0, which is the threshold for class III, showing that the river is slightly affected. The result of the MPI suggests that the river is slightly affected and possible sources of metal pollution includes discharges from diesel and petrol engine boats, the effluent from the cement bagging factory close to the sampling stations, activities of at the wharf of the Nigerian Ports Authority and discharge point close to the oil terminal and refinery effluent which passes through the dug-in channels from petroleum tank farm to the discharge points, which is the Bonny River. These are some of the major contributors whose signatures deteriorate the river water quality.
Statistical analysis of variance
Results of the physicochemical properties and trace metals were subjected to single-factor analysis of variance (ANOVA) using Microsoft Excel software.
The result of the single-factor ANOVA (Table 5) indicates that the sample sites show a significant effect on variation between groups of means for the physicochemical parameters and trace metals at different stations. It shows that the between-sample mean square is greater than the within-sample mean square, and the F test shows that this difference is highly significant. However, the mean trace metal concentrations of elements within sample sites differed significantly (F = 247.4565 > Fcrit = 1.70595; p < 0.05). The effects of the interaction between sample stations and trace metal concentration were highly significant, suggesting a transport phenomenon or mobility of the trace metals caused by activities such as Petroleum products transportation by oil vessels, countless outboard engine boats, maritime and oil-related activities, cement bagging factory, refinery effluent, industrial and municipal activities, and massive oil tankers within the wharf.
Conclusion
The data on water quality variables in the Bonny Estuary show seasonal variation with higher values for pH, DO, BOD, temperature, and salinity during the dry season than wet season. Levels of trace metals investigated revealed slightly higher values for most trace metals for all stations during the dry season than wet season. Results of metal pollution index were >1, which is the threshold showing that the river is slightly affected. Although the results of surface water concentrations of trace metals observed in the present study agrees with the general opinion of level trace metal concentrations in the study area and Niger Delta, some of the toxic metals such as Pb, Cd, Zn, Ni, and Cr were higher than stipulated limits by WHO (2006), and continuous monitoring is necessary to avert possible public health implications of these metals on consumers of water and seafood from the study area.
References
Adejuwon JO (2012) Rainfall seasonality in the Niger Delta Belt, Nigeria. J Geogr Reg Plan 5(2):51–60
Ajao EA, Fagade SO (2002) A study of the sediments and communities in Lagos, Lagoon. Oil Chem Pollut 7:85–117
Akaninwor JO, Anosike EO, Egwu O (2007) Effect of Indomie industrial effluent discharge on microbial properties of new Calabar River. Sci Res Essays 2:1–5
Amadi AN (2012) Quality assessment of Aba River using heavy metal pollution index. Am J Environ Eng 2(1):45–49
Amadi PA, Ofoegbu CO, Morrison T (1987) Hydrogeochemical assessment of groundwater quality in parts of the Niger Delta, Nigeria. Environ Geol Water Sci 14:195–202
Amajor LC (1991) Aquifers in the benin formation (Miocene–Recent), Eastern Niger Delta, Nigeria. Lithostratigraphy, hydraulics and water quality. Environ Geol Water Sci 17(2):85–101
Ansa EJ (2005) Studies of the Benthic Macrofauna of the Andoni Flats in the Niger Delta Area of Nigeria. Ph.D. Thesis University of Port Harcourt, Port Harcourt, Nigeria
Asonye CC, Okolie NP, Okenwa EE, Iwuanyanwu UG (2007) Some physico chemical characteristics and heavy metal profiles of Nigerian rivers, streams and waterways. Afr J Biotechnol 6(5):617–624
Caerio S, Costa MH, Ramos TB, Fernandes F, Silveira N, Coimbra A, Painho M (2005) Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol Ind 5:155–169
Chindah AC, Braide AS, Sibeudu OC (2004) Distribution of hydrocarbons and heavy metals in sediment and a crustacean (Penaeusnotialis) from the Bonny River/New Calabar River Estuary, Niger Delta. Afr J Environ Assess Manage 9:1–17
Doust H, Omatsola E (1990) Niger Delta. In: Edwards JD, Santogrossi PA (eds) Divergent/passive margin basins. AAPG Memoir 48. American Association of Petroleum Geologists, Tulsa, pp 239–248
Dublin-Green CO (1990) Seasonal variations in some physico-chemical parameters of the Bonny Estuary, Niger Delta. NIOMR Tech Pap 59:21–25
Durotoye B (1989) Quaternary sediments in Nigeria: In Kogbe (ed) Geology of Nigeria, rock view (Nigeria) Ltd. Publishers, Jos, pp 256
Eisler R (1993) Zinc hazards to fish, wildlife, and invertebrates: a synoptic review. US Fish Wildl Serv Biol Rep 10
Ekeh IB, Sikoki FD (2003) The state and seasonal variability in some physicochemical parameters of the New Calabar River, Nigeria. Suppl Ad Acta Hydrobiolog 5:45–60
Filatov N, Pozdnyakov D, Johannessen O, Pettersson L, Bobylev L (2005) White Sea: its marine environment and ecosystem dynamics influenced by global change. Springer and Praxis Publishing, UK, pp 1–472
Freda J (1991) The effects of aluminum and other metals on amphibians. Environ Pollut 71:305–328
Hospers J (1965) Gravity field and structure of the Niger Delta, Nigeria, West Africa. Geol Soc Am Bull 76:407–422
Igweze AH, Amagoh MN, Ashinze AN (2014) Analysis of Rainfall Variations in the Niger Delta Region of Nigeria. J Environ Sci Toxicol Food Technol 8(1):25–30
Jamabo N (2008) Aspects of the ecology of Tympanotonus fuscatus var fuscatus (Linnaeus 1758) in the Mangrove Swamps of the upper Bonny River. Niger Delta, Nigeria. Curr Res J Biol Sci 2:42–47
Kaplan A, Lusser CU, Norton IO (1994) Tectonic map of the world, panel 10. American Association of Petroleum Geologists, Tulsa
Klett TR, Ahlbrandt TS, Schmoker JW, Dolton JL (1997) Ranking of the world’s oil and gas provinces by known petroleum volumes. US Geological Survey Open-File Report 97–463
Kulke H (1995) Nigeria. In: Kulke H (ed) Regional petroleum geology of the world. Part II: Africa, America, Australia and Antarctica. Gebrüder Borntraeger, Berlin, pp 143–172
Lyulko I, Ambalova T, Vasiljeva T (2001) To integrated water quality assessment in Latvia. MTM (Monitoring Tailor-Made) III, proceedings of international workshop on information for sustainable water management. The Netherlands, pp 449–452
McLusky DS (1989) The estuarine ecosystem. Chapman and Hall, Glasgow
McNeely RN, Neimanis VP, Dwyer L (1979) Water quality sources book—a guide to water quality parameters. pp 112
Michele LWT, Ronald RC, Michael EB (1999) The Niger Delta Petroleum System: Niger Delta Province, Nigeria, Cameroon, and Equatorial Guinea, Africa. US Department of the Interior US Geological Survey. Open-File Report 99-50-H
Mohiuddin KM, Zakir HM, Otomo K, Sharmin S, Shikazono N (2001) Geochemical distribution of trace metal pollutants in water and sediments of downstream of an urban river. Int J Environ Sci Technol 7(1):17–28
Ofoegbu CO (1989) A review of the geology of the Benue Trough, Nigeria. J Afr Earth Sci 2:283–291
Oguzie FA (2002) Heavy metals in the sediments of the Lower Ikpoba River, Benin City, Nigeria. Sri Lanka J Aqua Sci 7:35–44
Onojake MC, Frank O (2013) Assessment of heavy metals in a soil contaminated by oil spill: a case study in Nigeria. Chem Ecol 29(3):246–254
Onojake MC, Ukerun SO, Iwuoha G (2011) A statistical approach for evaluation of the effects of industrial and municipal wastes on Warri Rivers, Niger Delta, Nigeria. Water Qual Expo Health 3:91–99
Saad MAH, Amuzu AT, Biney C, Calamari D, Imevbore AM, Neave H, Ocumba PBO (1994) Review of pollution in the African Aquatic environment; domestic and industrial organic loads. FAO CIFA Tech Pap 25:33–60
Sadiq M (1992) Toxic metal chemistry in marine environments. Marcel Dekker, New York
Sheikh MA, Noah NM, Tsuha K, Oomori T (2007) Occurrence of tributylin compounds and characteristics of heavy metals. Int J Environ Sci Technol E1:49–60
Sikoki FD, Zabbey N (2006) An aspect of fisheries of the middle reaches of Imo River, Niger Delta, Nigeria. Environ Ecol 24:309–312
Sindayigaya E, Cauwnbergh RV, Robberecht H, Deelstra H (1994) Copper, zinc, manganese, iron, lead, cadmium, mercury, and arsenic in fish from Lake Tanganyika, Burundi. Sci Total Environ 144:103–115
Short KC, Stauble AJ (1967) Outline of geology of Niger Delta. Am Assoc Pet Geol 51:761–779
Sundararaman C, Nicholas J, Diedjomahor JO (2002) Niger Delta Petroleum System: regional geology, organic facies and thermal maturity. AAPG annual meeting March 10–13, Houston Texas
Tamasi G, Cini R (2004) Heavy metals in drinking waters from Mount Amiata. Possible risks from arsenic for public health in the province of Siena. Sci Total Environ 327:41–51
Ubalua AO, Chijioke UC, Ezeronye OU (2007a) Determination and assessment of heavy metal content in fish and shellfish in Aba River, Abia state, Nigeria. KMITL Sci Tech J 7(1):31–45
Ubalua AO, Chijioke UC, Ezeronye OU (2007b) Determination and assessment of heavy metal content in fish and shellfish in Aba River, Abia state, Nigeria. KMITL Sci Tech J 7(1):31–45
United State Environment Protection Agency (1978) Quality criteria for water, EPA-440/9/76-023
Usero J, González-Regalado E, Gracia I (1997) Trace metals in the bivalve molluscsRuditapes decussatusand Ruditapes philippinarumfrom the Atlantic Coast of Southern Spain. Environ Int 23(3):291–298
WHO (2006) Guidelines for drinking-water quality (electronic resource). Incorporating first addendum. 3rd edn, volume 1. Recommendations. World Health Organization, Geneva
Zhou HG, Randers-Pehrson CA, Waldren CR, Geard DV, Eric JD, Hei TK (1999) Induction of Bystanders mutagenic effects of alpha particles in mammalian cells. Proc Natl Acad Sci 96:336–356
Zvinowanda CM, Okonkwo JO, Shabalala PN, Agyei NM (2009) A novel adsorbent for heavy metal remediation in aqueous environment. Int J Environ Sci Technol 6(3):425–434
Acknowledgments
The authors are grateful to the International Atomic Energy Agencies (IAEA) IAEA-FGN Project, RAF/7009 for their funding and support that brought this research to fruition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Onojake, M.C., Sikoki, F.D., Omokheyeke, O. et al. Surface water characteristics and trace metals level of the Bonny/New Calabar River Estuary, Niger Delta, Nigeria. Appl Water Sci 7, 951–959 (2017). https://doi.org/10.1007/s13201-015-0306-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0306-y