Abstract
The capability of Jordanian natural zeolite to remove nickel from aqueous solutions was experimentally investigated using a packed bed column. The zeolite samples were obtained from Jabal AL Aritayn in the northeast of Jordan. The effects of the initial concentration of nickel (C0), the packed bed length (L) and the zeolite grain size (Dp) on the adsorption process were considered. The finding indicated that these parameters named had a significant effect on the removal of nickel by the Jordanian zeolite. The characteristic breakthrough curves of the adsorption process were measured. The Klinkenberg model was adopted to explain the kinetic behavior of the adsorption phenomena, and we attempted to fit the packed bed experimental data to the breakthrough curve. The effective diffusivity was estimated and used to predict breakthrough curves under other adsorption conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of heavy metals in industrial wastewater as a result of many manufacturing process is known to cause detrimental effects on human health and environment. Removing heavy metals demands high energy or advanced operational requirements. A number of conventional technologies such as coagulation, precipitation, ion-exchange, electrochemical methods, membrane processes, extraction, biosorption, and adsorption have been considered for the treatment of contaminated wastewater (Wang and Peng 2010; Babel and Kurniawan 2003; Kwon et al. 2010). Among these methods, adsorption is found to be very suitable for wastewater treatment because of its simplicity and cost effectiveness (Yadanaparthi et al. 2009; Argun 2008; Panayotova 2001). Commercial activated carbon is regarded as the most effective material for controlling the metal ions load. However, due to its high cost and 10–15 % loss during regeneration, unconventional adsorbents like sand, alumina, silica, and zeolite have attracted the attention of several investigations and adsorption characteristics and have been widely investigated for the removal of metal ions (Bailey et al. 1999; Babel and Kurniawan 2003). Nowadays, natural zeolite found in many environmental applications, which gain the attention of many researchers due to its properties such as proprieties adsorption, catalysis and ion exchange (Weng and Huang 1994; Erdem et al. 2004; Benefield et al. 1982; Adrian 1948; Zhang 2006).
Zeolites are a group of hydrated aluminum silicates of the alkali or alkaline earth metals (sodium, potassium, magnesium, calcium) characterized by low mining cost, availability, bulk density and high resistance to alteration (Mercer and Ames 1978). Zeolites have a three-dimensional crystalline framework of tetrahedral silica or alumina anions strongly bonded at all corners and they contain channels filled with water and exchangeable cations. Ion exchange capacity and cation selectivity are the most important properties for zeolite as a natural molecular sieve material for wastewater treatment. Cation selectivity refers to the preference order of zeolite for cations based on the various factors which determine the selectivity. Ion size, valence and hydration energies are important factors in determining selectivity of a given ion in specific system. The zeolite prefers or is more selective for certain cations and less selective for others (Colella 1996).
In general, a packed-bed column is preferable over a batch adsorber because of its ability to process large quantities of feed under continuous operation. The design of an industrial packed-bed column requires many parameters, which can be obtained by doing a series of laboratory experiments. However, such practices are time consuming and costly so that accurate modeling and simulation are frequently used as an alternative for predicting the dynamic behavior of packed bed systems to optimize column design and operation parameters.
Purnomo and Prasetya (2007) studied the adsorption breakthrough curves of Cr(VI) on bagasse fly ash. They measured the breakthrough curve at room temperature using a fixed-bed apparatus. They tried to fit the experimental data to fixed-bed model for breakthrough curve. They concluded that if the value of De and k can be obtained, it will allow an easier prediction of the behavior of breakthrough curves in a specific adsorption operating conditions and column dimensions without doing any adsorption equilibrium experiments.
Nevenka et al. (2010) studied the natural zeolite from Serbian as adsorbent for nickel ions from aqueous solution. They found that the sorption capacity increased three times by increasing the temperature from 298 to 338 K. The sorption was best described by the Sips isotherm model. This was in accord with observations generally encountered in heavy metal sorption studies (Alyuz and Veli 2009; Lu et al. 2009; Hsu 2009). They observed that the sorption involved a film diffusion, an intra-particle diffusion, and a chemical cation-exchange between the Na+ ions of zeolite and the Ni2+ ions.
Chang et al. (2006) studied experimentally the adsorption isotherms of water vapor on cornmeal and the breakthrough curves in a packed-bed apparatus for ethanol dehydration. The effective diffusivity was estimated and used to predict breakthrough curves at other adsorption conditions.
In addition, number of studies have been conducted on the adsorption behavior of heavy metal on Jordanian natural Zeolitic Tuff (Al Dwairi 2009; Baker et al. 2009). However, there has been no attempt to correlate them with equilibrium and kinetic information for prediction of breakthrough. In this work, our aim is to measure the nickel isotherms in a packed bed of Jordanian Zeolitic Tuff. By fitting the experimental data to model of breakthrough curve, the effective diffusivity of nickel was estimated. Another sub goal of this study is to investigate the effect of using different grain size and bed length of Zeolitic Tuff on the adsorption capacity and the behavior of the experimental and numerical breakthrough curves and their matching percentage.
Materials and methods
Zeolitic tuff used in the present study was obtained from Jabal Al Aritayn in northeast Jordan. The bulk sample was sieved into three different grain sizes (Table 1) and washed by distilled water to remove surface dust. Then it had been dried at 120 ºC for 12 h. The model of SEM used is shown in Fig. 1). Chemical composition of zeolite samples was determined by the usual analytical methods for silicate materials. Standard wet chemical analysis along with instrumental methods was adopted (Corbin et al. 1987).
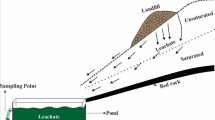
The ion exchange of nickel on Jordanian natural zeolite was carried out using the packed bed column (Fig. 2). The packed bed adsorption experiments were conducted using columns made of glass with 60 cm height and 5 cm2 base area. The solutions containing heavy metal ions used in this experiments were prepared by spiking a known concentration of nickel ions ranging from 20 to 100 mg/L at a constant temperature of 24 °C. The exact concentration of metal ions was determined using an atomic absorption spectrometer (Pye Unicam SP9 Cambridge, UK). The effect of grain size on the adsorption of nickel ions was studied at a fixed bed height of 40 and 20 cm for inlet concentration 20 mg/L.
Mathematical models
Linear adsorption isotherm
Assuming linear adsorption according to Henry’s law for dilute concentration of nickel, the adsorption isotherm for nickel has been correlated as a linear adsorption isotherm over the concentration range from 20 to 100 mg/L at 24 ºC, which is given by,
where K is the adsorption equilibrium constant for a linear adsorption isotherm, q denotes the mass of nickel adsorbed in mg/g adsorbent and c is the concentration of nickel in mg/L.
Model for breakthrough curve
The aim of the experiments is to predict mass transfer diffusivity and breakthrough curves from the measured adsorption isotherm. The model is developed from mass balance on the solute for the flow of solution through a differential adsorption-bed length, dZ over a differential time, dt. Thus, the generated equation gives the concentration of solute in the bulk fluid as a function of time and location in the bed.
In Eq. (2) the first term represents the axial dispersion with eddy diffusion DL, the second terms account an axial variation in fluid velocity, and the fourth term that is based on q, throughout the adsorbent grain. The linear driving force model for mass transfer Seader and Henley (2002) is given by:
where \( q{}^{*} \) is the adsorbate loading in equilibrium with the solute concentration c, in the bulk fluid. \( c{}^{*} \) is the concentration in equilibrium with average loading q; k is the overall mass-transfer coefficient in s−1, which includes both external and internal transport resistances; and K is the adsorption equilibrium constant for a linear adsorption isotherm in Eq. (1). For estimating several variables, the value can be obtained by employing the two following equations (Seader and Henley, 2002).
where kc is the external mass-transfer coefficient in m/s, De is effective diffusivity in m2/s and Rp is adsorbent grain radius in m. The first term in Eq. (4) is the overall mass-transfer resistances, the second and third terms are external and internal ones, respectively. The external transport coefficient of grains in packed-bed can be correlated by Seader and Henley (2002).
where Sh = Sherwood number = KcDp/Di, Re = Reynolds number = DpG/μ, and Sc i = Schmidt number = μ/ρDi. By Eq. (5), external mass-transfer coefficient kc can be estimated from Sh.
The analytical solution of Eq. (2), in which constant fluid velocity u, negligible axial dispersion, and the linear driving force mass-transfer model were assumed, was provided by Kinkenberg. An adopted approximate solution is represented below:
where \( \xi = (kKZ/u)((1 - \varepsilon_{\text{b}} )/\varepsilon_{\text{b}} ) \) is dimensionless distance coordinate, \( \tau = k(t - (Z/u)) \) the dimensionless time coordinate and erf(x) is error function that is defined as:
ξ and τ are coordinate transformations for Z and t, and the equations are converted to a much simpler form of erf(x). The approximation given by Eq. (6) is known to be acceptable and the error could be within 0.6 % for ξ > 2.0.
Klinkenberg model has also included the following approximate solution for the profiles of solute concentration in equilibrium with the average sorbent loading:
where \( c{}^{*} = \bar{q}/K \) and \( c^{ * } /c_{\text{F}} = \bar{q}/q_{\text{F}}^{ * } \) where \( q_{\text{F}}^{ * } \) is the loading in equilibrium with cF.
The simple model of Klinkenberg, giving \( c/c_{\text{F}} \) as a function of dimensionless time τ, and dimensionless bed length ξ, was not worse than more sophisticated models, and could be known as adequate for preliminary design purpose (Chang et al. 2006).
The equilibrium constant K at constant temperature can be correlated by experimental data. By fitting the experimental breakthrough curves to Eq. 6, the overall mass-transfer coefficient k can be estimated. Then by Eqs. 4 and 5, the effective diffusivity De can be evaluated. In addition, the breakthrough curves at various conditions can be predicted by De which has been obtained.
Results and discussion
The adsorption isotherm of nickel is shown in Fig. 3. By fitting the experimental results of adsorption isotherm to the linear isotherm represented by Eq. 1, the adsorption equilibrium constant at ambient temperatures was correlated. From Fig. 3, it can be seen that the linear correlation represents the isotherm fairly well with the value of the correlation coefficient R2 as 0.989. The linear adsorption isotherms for nickel were used in the prediction of breakthrough curves. Figure 3 shows also the theoretical adsorption capacity of Jordanian zeolite compared with the Serbian zeolite under the same conditions. It is clear that the Jordanian zeolite is more efficient in removing nickel ions.
The fitting method between experiment and calculation data was carried out using numerical method of error minimization by sum of square error (SSE) principle. The chosen numerical method was multi variables minimization. First, the value of k and ξ was guessed and obtained as (ci/cF) in Eq. 6 and then iterated to get lowest SSE. The other variables in Eq. 6 such as bed depth Z, Equilibrium isotherm constant K, the bed void fraction εb, and constant velocity u are known. The best obtained value of the both variables can be used to determine De by Eqs. 4 and 5. The values of k, ξ and calculated De are listed in Table 1. From the table, the overall mass transfer coefficient obtained at different grain size and fixed bed height can be used to predict well the breakthrough curves. It can be note that the overall mass transfer coefficient is similar for different grain size and bed height due to the fact that internal mass transfer is important factor. As a result, the average estimated value for De is in the order of 5.63 × 10−12 m2 s−1.
The comparison of correlation breakthrough curve for adsorption of nickel on Jordanian Zeolite with experimental data by varying the grain size and different fixed bed height is shown in Figs. 4 and 5. From Fig. 4, it can be seen that the values of k as 1.66 × 10−3 s−1, 1.754 × 10−3 s−1 and 1.89 × 10−3 s−1 can be applied to well predict the breakthrough curves for adsorption of nickel on Jordanian zeolite at a fixed bed height of 40 cm using numerical method of error minimization by sum of square error (SSE) principle. This indicates that Klinkenberg’s model is quite adequate for our experimental conditions, and that it is valuable for industrial applications, i.e., direct measurement on a small sample can be used to predict the breakthrough curves of a larger absorber by means of the numerical solution of the model.
It can be also noted form Fig. 5 that the values of k as 1.61 × 10−3 s−1, 1.70 × 10−3 s−1 and 1.754 × 10−3 s−1 can be applied to well predict the breakthrough curves for different grain size at a fixed bed height of z = 20 cm. It can be noted that the value of De remains constant for the two different columns under the same temperature, using the same grain size. Meanwhile, the values of k slightly increase by decreasing the grain size and increasing the fixed bed height due to the increase in surface area of the adsorbent.
Figures 4 and 5 show the effect of grain size on the adsorption of nickel ions by varying the grain size at fixed bed height 40 and 20 cm, respectively. In general, the breakthrough curves became steeper and the breakthrough time decreased with increasing the grain size. This behavior may be due to insufficient residence time of the solute in the column and diffusion limitations of the solute into the pores of the sorbent at bigger grain size.
It can be seen from Fig. 4 that for fixed bed height of 40 cm, the concentration of nickel ions appears in effluent solution after 149, 268 and 510 min for 0.48, 0.42 and 0.37 bed porosity, respectively. Meanwhile, the concentration of nickel in effluent solution reaches the saturation point after 417, 620 and 970 min for 0.48, 0.42 and 0.37 bed porosity, respectively. The concentration of nickel ions appears in effluent solution for fixed bed height of 20 cm after 85, 155 and 430 min for 0.048, 0.42 and 0.37 bed porosity, respectively. Meanwhile, the concentration of nickel in effluent solution reaches the saturation point after 340, 410 and 830 min for 0.48, 0.42 and 0.37 bed porosity, respectively. As the bed porosity increases, the breakpoint time decreases due to the fact that both the binding sites and ion exchange sites become quickly saturated. As we predict, the decrease in the bed porosity or increases the fixed bed height lead to increase in the contacting time between the ions and the adsorbent which in turns increases the saturation point.
To study the removal efficiency of nickel ions from aqueous solution using two different bed heights and to compare between them, the results were plotted as breakthrough curves for the same bed porosity as shown in Fig. 6. From this figure, one can notice that the uptake of nickel increased with an increase in bed height. The reason behind the increase in the uptake capacity of nickel ions as bed height increases is availability of more binding sites for sorption, which also resulted in a broadened mass transfer zone.
Conclusion
The adsorption isotherms for nickel at ambient temperature were measured in a packed-bed apparatus. The nickel isotherms were linearized and applied to the prediction of breakthrough curves. By fitting the experimental results of breakthrough curves to the model of Klinkenberg, the overall mass-transfer coefficient was estimated and successfully used for the prediction of breakthrough curves at different bed porosity and different bed depth. The experimental results showed that for a given length, the break point time decreases with increasing zeolite grain size and the breakthrough curve becomes steeper. Decreasing the bed height the break point is obtained earlier which represents the realistic behavior in the adsorption phenomena. It is also found that the Klinkenberg model parameters include mass transfer coefficients (k) and effective diffusivities (De) can be estimated independently of the experimental data. The model is able to predict the breakthrough curves accurately.
Abbreviations
- c :
-
Concentration of nickel (g/L)
- c F :
-
Concentration of nickel in effluent (g/L)
- \( c^{*} \) :
-
Concentration in equilibrium with average loading (g/L)
- D i :
-
Molecular diffusivity (m2/s)
- D p :
-
Diameter of adsorbent (m)
- erf(x):
-
Error function
- k :
-
Overall mass transfer coefficient (s−1)
- k c :
-
External mass transfer coefficient (m/s)
- K :
-
Adsorption equilibrium constant
- q :
-
Adsorption capacity (mg/g)
- \( \bar{q} \) :
-
Average loading of adsorbent (mg/g)
- \( q{}^{*} \) :
-
Adsorbate loading in equilibrium (mg/g)
- Re :
-
Reynolds number
- R p :
-
Radius of adsorbent (m)
- Sc i :
-
Schmidt number
- Sh :
-
Sherwood number
- t :
-
Time (s)
- u :
-
Effective velocity (m/s)
- u 0 :
-
Superficial velocity (m/s)
- μ:
-
Viscosity (Pa s)
- ξ:
-
Dimensionless distance coordinate
- ρ:
-
Density (kg/m3)
- τ:
-
Dimensionless time coordinate
- ε:
-
Bed porosity
References
Adrian K (1948) Numerical evaluation of equations describing transient heat and mass transfer in packed solids. Ind Eng Chem 40:1992–1994
Al Dwairi R (2009) The use of expendable local zeolite deposits for NH4 removal in municipal wastewater. Jordan J Civil Eng 3:256–264
Alyuz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solution by ion exchange resins. J Hazard Mater 167:482–488
Argun ME (2008) Use of clinoptilolite for the removal of nickel ions from water: kinetics and thermodynamics. J Hazard Mater 150:587–595
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J. Hazard Mater B 97(219):243
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low cost sorbents for heavy metals. Water Res 33:2469–2479
Baker H, Massadeh A, Younes H (2009) Natural Jordanian zeolite: removal of heavy metal ions from water samples using column and batch methods. Environ Monit Assess 157:319–330
Benefield L, Judkins J, Weaned B (1982) Process chemistry for water and wastewater treatment. Prentice-Hall, USA
Chang H, Yuan X, Tian H, Zeng A (2006) Experiment and prediction of breakthrough curves for packed bed adsorption of water vapor on cornmeal. Chem Eng Process 45:747–754
Colella C (1996) Ion exchange equilibria in zeolites minerals, mineral. Deposita 31:554–562
Corbin DR, Burgess BF, Vega AJ, Farelee RD (1987) Comparison of analytical techniques for the determination of silicon and aluminium content in zeolites. Anal Chem 59:2722–2728
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Coll Inter Sci 280:309–314
Hsu TC (2009) Experimental assessment of adsorption of Cu2+ and Ni2+ from aqueous solution by oyster shell powder. J Hazard Mater 171:995–1000
Kwon JS, Yun ST, Lee JH, Kim SO, Jo HY (2010) Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic(III) from aqueous solutions using scoria:kinetics and equilibria of sorption. J Hazard Mater 174:307–313
Lu C, Liu C, Su F (2009) Sorption kinetics, thermodynamics and competition of Ni2+ from aqueous solutions onto surface oxidized carbon nanotubes. Desalination 24:18–23
Mercer BW, Ames LL (1978) Zeolite ion exchange in radioactive and municipal wastewater treatment. In: Sand LB, Mumpton FA (eds) Natural zeolites: occurrence, properties, use. Pergamon Press, Elmsford, pp 451–462
Nevenka R, Djordje S, Mina J, Natasa ZL, Matjaz M, Venceslav K (2010) Removal of nickel(II) ions from aqueous solutions using the natural clinoptilolite and preparation of nano-NiO on the exhausted clinoptilolite. Appl Surf Sci 257:1524–1532
Panayotova M (2001) Kinetics and thermodynamics of removal of nickel ions from wastewater by use of natural and modified zeolite, Fresen. Environ Bull 10:267–272
Purnomo CW, Prasetya, A (2007) The study of adsorption breakthrough curves of Cr(VI) on Bagasse Fly Ash (BFA). In: Proceedings of the world congress on engineering and computer science, San Francisco, USA
Seader JD, Henley EJ (2002) Separation process principles, John Wiley&Sons, Inc., New York
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24
Weng CH, Huang CP (1994) Treatment of metal industrial wastewater by fly ash and cement fixation. J Environ Eng 120:1470–1487
Yadanaparthi SKR, Graybill D, Wandruszka R (2009) Adsorbents for the removal of arsenic, cadmium, and lead from contaminated waters. J Hazard Mater 171:1–15
Zhang YS (2006) Development of heavy metal adsorbed by granulation of natural zeolite, 18th World Congress of Soil Science, Philadelphia, PA, USA
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Taamneh, Y., Al Dwairi, R. The efficiency of Jordanian natural zeolite for heavy metals removal. Appl Water Sci 3, 77–84 (2013). https://doi.org/10.1007/s13201-012-0061-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0061-2