Abstract
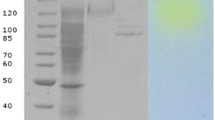
The β-galactosidase is an industrially valuable enzyme and used to hydrolyze the lactose into glucose and galactose. Considering the broad utility profile in food industry, β-galactosidase from Aspergillus nidulans was purified and characterized in term of its catalytic properties and stability. It displayed highest catalytic efficiency at 60 °C after 10.0 min within acidic pH environment (pH 5). The β-galactosidase exhibited 100% and 60% catalytic activity at 40 °C and 50 °C, respectively even after 120.0 min. The β-galactosidase activity was remained stable in the presence of Zn2+, Ni2+, and Mg2+ ions. The activity was also retained in all investigated organic solvents except DMSO at various ionic concentrations. The surfactants Triton X-100 and SDS caused positive impact on the catalytic activity of enzyme at 1.0 mM concentration. However, the percent relative activity of β-galactosidase was significantly reduced when incubated with EDTA. The molecular mass of β-galactosidase estimated to be 95 kDa. The SEM micrographs of ONPG before and after β-galactosidase treatment indicated a remarkable difference in the morphology and proved the strong catalytic strength of enzyme. The β-galactosidase also demonstrated exceptional storage stability at − 80 °C, − 20 °C and 4 °C by retaining 86, 79 and 70% activity even after 100.0 days.



Similar content being viewed by others
References
Alikkunju AP, Sainjan N, Silvester R, Joseph A, Rahiman M, Antony AC, Kumaran RC, Hatha M (2016) Screening and characterization of cold-active β-galactosidase producing psychrotrophic Enterobacter ludwigii from the sediments of Arctic Fjord. Appl Biochem Biotechnol 180:477–490. https://doi.org/10.1007/s12010-016-2111-y
Bekler FM, Yalaz S, Acer O, Guven K (2017) Purification of thermostable β-galactosidase from Anoxybacillus sp. KP1 and estimation of combined effect of some chemicals on enzyme activity using semiparametric errors in variables model. Fresenius Environ Bull 26:2251–2259
Bhatti HN, Asgher M, Abbas A, Nawaz R, Sheikh MA (2006) Studies on kinetics and thermostability of a novel acid invertase from Fusarium solani. J Agric Food Chem 54:4617–4623
Chanalia P, Gandhi D, Attri P, Dhanda S (2018) Purification and characterization of β-galactosidase from probiotic Pediococcus acidilactici and its use in milk lactose hydrolysis and galactooligosaccharide synthesis. Bioorg Chem 77:176–189
Cieśliński H, Kur J, Białkowska A, Baran I, Makowski K, Turkiewicz M (2005) Cloning, expression, and purification of a recombinant cold-adapted β-galactosidase from Antarctic bacterium Pseudoalteromonas sp. 22b. Protein Expr Purif 39:27–34
Dako E, Dadie AT, Bernier A-M, Jankowski CK (2012) The problems associated with enzyme purification. In: Ekinci D (ed) Chemical biology. INTECH Open Access Publisher, London, pp 19–40
Fischer L, Scheckermann C, Wagner F (1995) Purification and characterization of a thermotolerant beta-galactosidase from Thermomyces lanuginosus. Appl Environ Microbiol 61:1497–1501
Harju M, Kallioinen H, Tossavainen O (2012) Lactose hydrolysis and other conversions in dairy products: technological aspects. Int Dairy J 22:104–109
Hecht MH (1996) Strategies for the design of novel proteins. In: Carey P (ed) Protein engineering and design. Academic Press, New York, pp 1–50
Hung MN, Lee B (2002) Purification and characterization of a recombinant β-galactosidase with transgalactosylation activity from Bifidobacterium infantis HL96. Appl Microbiol Biotechnol 58:439–445
Jiang Z, Cong Q, Yan Q, Kumar N, Du X (2010) Characterization of a thermostable xylanase from Chaetomium sp. and its application in Chinese steamed bread. Food Chem 120:457–462
Juajun O, Nguyen T, Maischberger T, Iqbal S, Haltrich D, Yamabhai M (2011) Cloning, purification, and characterization of β-galactosidase from Bacillus licheniformis DSM 13. Appl Microbiol Biotechnol 89:645–654
Kamran A, Bibi Z, Aman A, Qader SAU (2017) Hyper production of β-galactosidase from newly isolated strain of Aspergillus nidulans. J Food Proc Eng 40:12452. https://doi.org/10.1111/jfpe.12452
Karan R, Capes MD, DasSarma P, DasSarma S (2013) Cloning, overexpression, purification, and characterization of a polyextremophilic β-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi. BMC Biotechnol 13:3. https://doi.org/10.1186/1472-6750-13-3
Karasová-Lipovová P, Strnad H, Spiwok V, Malá Š, Králová B, Russell NJ (2003) The cloning, purification and characterisation of a cold-active β-galactosidase from the psychrotolerant Antarctic bacterium Arthrobacter sp. C2-2. Enzyme Microb Technol 33:836–844
Kurniati A, Darmokoesoemo H, Puspaningsih NNT (2016) Scanning electron microscope analysis of rice straw degradation by a treatment with α-L-arabinofuranosidase. Proc Chem 18:63–68
Lee DH, Kang SG, Suh SG, Byun JK (2003) Purification and characterization of a beta-galactosidase from peach (Prunus persica). Mol Cells 15:68–74
Li Y, Wang H, Lu L, Li Z, Xu X, Xiao M (2009) Purification and characterization of a novel β-galactosidase with transglycosylation activity from Bacillus megaterium 2-37-4-1. Appl Biochem Biotechnol 158:192–199
Liao X, Zheng Q, Zhou Q, Lin J, Guo L, Yun F (2016) Characterization of recombinant β-galactosidase and its use in enzymatic synthesis of lactulose from lactose and fructose. J Mol Catal B Enzym 134:253–260
Liu Z, Zhao C, Deng Y, Huang Y, Liu B (2015) Characterization of a thermostable recombinant β-galactosidase from a thermophilic anaerobic bacterial consortium YTY-70. Biotechnol Biotechnol Equip 29:547–554. https://doi.org/10.1080/13102818.2015.1015244
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–375
Lozano JE (2006) Processing of fruits: ambient and low temperature processing. In: Fruit manufacturing. Springer, Boston, MA, pp. 21–54. https://doi.org/10.1007/978-0-387-30616-2_2
Mlichová Z, Rosenberg M (2006) Current trends of β-galactosidase application in food technology. J Food Nutr Res 45:47–54
Nagy Z, Kiss T, Szentirmai A, Biró S (2001) β-Galactosidase of Penicillium chrysogenum: production, purification, and characterization of the enzyme. Protein Expr Purif 21:24–29
Nath A, Chakrabarty S, Sarkar S, Bhattacharjee C, Drioli E, Chowdhury R (2013) Purification and characterization of β-galactosidase synthesized from Bacillus safensis (JUCHE 1). Ind Eng Chem Res 52:11663–11672
Nawaz MA, Karim A, Aman A, Marchetti R, Qader SA, Molinaro A (2015) Continuous degradation of maltose: improvement in stability and catalytic properties of maltase (α-glucosidase) through immobilization using agar-agar gel as a support. Bioprocess Biosyst Eng 38:631–638
Onishi N, Tanaka T (1995) Purification and properties of a novel thermostable galactooligosaccharide-producing betagalactosidase from Sterigmatomyces elviae (CBS8119). Appl Environ Microbiol 61:4026–4030
Pan SQ, Ye XS, Kue J (1989) Direct detection of β-1,3-glucanase isozymes on polyacrylamide electrophoresis and isoelectrofocusing gels. Anal Biochem 182:136–140
Pereira-Rodríguez Á, Fernández-Leiro R, González-Siso MI, Cerdán ME, Becerra M, Sanz-Aparicio J (2012) Structural basis of specificity in tetrameric Kluyveromyces lactis β-galactosidase. J Struct Biol 177:392–401
Qader SAU, Aman A, Syed N, Bano S, Azhar A (2007) Characterization of dextrasucrase immobilized on calcium alginate beads from Leuconostoc mesenteroides PCSIR-4. Ital J Biochem 56:158–162
Raol GG, Raol BV, Prajapati VS, Patel KC (2015) Kinetic and thermodynamic characterization of a halotolerant β-galactosidase produced by halotolerant Aspergillus tubingensis GR1. J Basic Microbiol 54:1–11
Rehman HU, Aman A, Silipo A, Qadar SAU, Molinaro A, Ansari A (2013) Degradation of complex carbohydrate: immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Food Chem 139:1081–1086
Rehman HU, Aman A, Nawaz MA, Qader SAU (2015) Characterization of pectin degrading polygalacturonase produced by Bacillus licheniformis KIBGE-IB21. Food Hydrocoll 43:819–824
Sako T, Matsumoto K, Tanaka R (1999) Recent progress on research and applications of nondigestible galactooligosaccharides. Int Diary J 9:69–80
Sen S, Ray L, Chattopadhyay P (2012) Production, purification, immobilization, and characterization of a thermostable β-galactosidase from Aspergillus alliaceus. Appl Biochem Biotechnol 167:1938–1953. https://doi.org/10.1007/s12010-012-9732-6
Song C, Liu G, Xu J, Chi Z (2010) Purification and characterization of extracellular β-galactosidase from the psychrotolerant yeast Guehomyces pullulans 17-1 isolated from sea sediment in Antarctica. Process Biochem 45:954–960
Wu H, Cheng X, Zhu Y, Zeng W, Chen G, Liang Z (2018) Purification and characterization of a cellulase-free, thermostable endo-xylanase from Streptomyces griseorubens LH-3 and its use in biobleaching on eucalyptus kraft pulp. J Biosci Bioeng 125:46–51. https://doi.org/10.1016/j.jbiosc.2017.08.006
Yang J, Di X, Wang M, Gao R (2015) Gene clone and characterization of a novel thermostable β-galactosidase with transglycosylation activity from Thermotoga naphthophila RUK-10. Chem Res Chin Univ 31:564–568
Acknowledgements
This research work was financially supported by the Karachi Institute of Biotechnology and Genetic Engineering (KIBGE), University of Karachi, Karachi-75270, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamran, A., Bibi, Z., Aman, A. et al. Purification and catalytic behavior optimization of lactose degrading β-galactosidase from Aspergillus nidulans. J Food Sci Technol 56, 167–176 (2019). https://doi.org/10.1007/s13197-018-3470-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3470-x