Abstract
Purpose
Lymph node (LN) characterization is crucial in determining the stage and treatment decisions in patient with lung cancer. Although 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has a higher diagnostic accuracy in LN characterization than anatomical imaging, differentiating between metastatic and inflammatory LNs is still challenging because both could show high 18F-FDG uptake. The purpose of this study was to assess if the heterogeneity of the 18F-FDG uptake could help in differentiating between inflammatory and metastatic LNs in lung cancer, and to compare with other parameters.
Methods
A total of 44 patients with adenocarcinoma of the lung, who underwent preoperative 18F-FDG PET/CT without having any previous treatments and were revealed to have 18F-FDG-avid LNs, were enrolled. There were 52 pathology-proven metastatic lymph nodes in 26 subjects. The pathology-proven metastatic LNs were compared with 42 pathology-proven inflammatory/benign LNs in 18 subjects. The coefficient of variation (CV) was used to assess the heterogeneity of 18F-FDG uptake by dividing the standard deviation of standardized uptake value (SUV) by mean SUV. The volume of interest was manually drawn based on the combined CT images of 18F-FDG PET/CT (no threshold is used). Comparisons were made with the maximum standardized uptake values (SUVmax), visual assessment of 18F-FDG uptake, longest diameter, and maximum Hounsfield units (HUmax).
Results
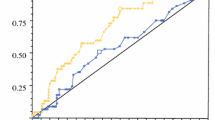
Metastatic lymph nodes tended to have higher CVs than the inflammatory LNs. The mean CV of metastatic LNs (0.30 ± 0.08; range: 0.08–0.55) was higher than that of inflammatory LNs (0.17 + 0.06; range, 0.07–0.32; P < 0.0001). On receiver operating characteristic (ROC) curve analysis, the area under curve was 0.901, and using 0.20 as cut-off value, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were 88.5 %, 76.2 %, 82.2 %, 84.3, and 83.0 % respectively. Accuracy of CV was slightly higher than SUVmax and diameter, but significantly higher than visual assessment and HUmax.
Conclusions
In patients with adenocarcinoma of the lung having no prior treatments, metastatic LNs showed more heterogeneous 18F-FDG uptake than inflammatory LNs. Measuring the CV of the SUV derived from a manual volume of interest (VOI) can be helpful in determining metastatic LN of adenocarcinoma of the lung. Including diagnostic criteria of CV into the diagnostic approach can increase the accuracy of mediastinal node status.






Similar content being viewed by others
References
Pieterman RM, Van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koeter GH, et al. Preoperative staging of non-small cell lung cancer with positron-emission tomography. N Eng J Med. 2000;343:254–61.
De Leyn P, Vansteenkiste J, Cuypers P, Deneffe G, Van Raemdonck D, Coosemans W, et al. Role of cervical mediastinoscopyin staging of non-small cell lung cancer without enlarged mediastinal lymph nodes on CT scan. Eur J Cardiothorac Surg. 1997;12:706–12.
Fischer B, Lassen U, Morten J, Larsen S, Loft A, Bertelsen A, et al. Preoperative staging of lung cancer with combined PET-CT. N Eng J Med. 2009;361:32–9.
Perigaud C, Bridji B, Roussel JC, Sagan C, Mugniot A, Duveau D, et al. Prospective preoperative mediastinal lymph node staging by integrated positron emission tomography-computerised tomography in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg. 2009;36:731–6.
Yang W, Fu Z, Yu J, Yuan S, Zhang B, Li D, et al. Value of PET/CT versus enhanced CT for locoregional lymph nodes in non-small cell lung cancer. Lung Cancer. 2008;61(1):35–43.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30.
Chung JH, Cho KJ, Lee SS, Baek HJ, Park JH, Cheon GJ, et al. Overexpression of Glut1 in lymphoid follicles correlates with false-positive 18F-FDG PET results in lung cancer staging. J Nucl Med. 2004;45:999–1003.
Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Active and passive mechanisms of [fluorine-18]fluorodeoxyglucose uptake by proliferating and prenecrotic cancer cells in vivo: A microautoradiographic study. J Nucl Med. 1994;35:1067–75.
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucosein vivo: High accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–80.
Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Whal RL. Intratumoral distribution of tritiatedfluorodeoxyglucose in breast carcinoma. I. Are inflammatory cells important? J Nucl Med. 1995;36:1854–61.
Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: Histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16.
Higashi K, Anaira CC, Wahl RL. Does FDG uptake measure proliferative activityof human cancer cells? In vitro comparison with DNA flow cytometry and tritiated thymidine uptake. J Nucl Med. 1993;34:414–9.
Hellwig D, Graeter TP, Ukena D, Groeschel A, Sybrecht GW, Schaefers HJ, et al. 18F-FDG PET for mediastinal staging of lung cancer: Which SUV threshold makes sense? J Nucl Med. 2007;48:1761–6.
Kernstine KH, Stanford W, Mullan BF, Rossi NP, Thompson BH, Bushnell DL, et al. PET, CT, and MRI with Combidex for mediastinal staging in non-small cell lung carcinoma. Ann Thorac Surg. 1999;68:1022–8.
Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: A prospective trial. Ann Thorac Surg. 2005;80:1207–13.
Cho YS, Choi JY, Lee KS, Kwon OJ, Shim YM, Lee SJ, et al. FDG PET/CT criteria for diagnosing mediastinal lymph node metastasis in patients with non-small cell lung cancer. J Nucl Med. 2007;48 (Suppl 2):85P.
Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis: Correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8.
Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64.
Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–8.
Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58.
Vamesu S. Angiogenesis and tumor grading in primary breast cancer patients: An analysis of 158 needle core biopsies. Rom J Morphol Embryol. 2006;47:251–7.
Groves AM, Shastry M, Rodriguez-Justo M, Malhotra A, Endozo R, Davidson T, et al. 18F-FDG PET and biomarkers for tumour angiogenesis in early breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:46–52.
Guo J, Higashi K, Ueda Y, Oguchi M, Takegami T, Toga H, et al. Microvessel density: Correlation with 18F-FDG uptake and prognostic impact in lung adenocarcinomas. J Nucl Med. 2006;47:419–25.
Vakkila J, Lotze MT. Inflammation and necrosis promote tumourgrowth. Nat Rev Immunol. 2004;4:641–8.
Nguyen XC, So Y, Chung JH, Lee WW, Park SY, Kim SE. High correlations between primary tumours and loco-regional metastatic lymph nodes in non-small-cell lung cancer with respect to glucose transporter type 1-mediated 2-deoxy-2-F18-fluoro-D-glucose uptake. Eur J Cancer. 2008;44:692–8.
Takamochi K, Yoshida J, Murakami K, Niho S, Ishii G, Nishimura M, et al. Pitfalls in lymph node staging with positron emission tomography in non-small cell lung cancer patients. Lung Cancer. 2005;47:235–42.
Van der Valk P, Meijer CJ. The histology of reactive lymph nodes. Am J Surg Pathol. 1987;11:866–82.
Scott WJ, Gobar LS, Terry JD, Dewan NA, Sunderland JJ. Mediastinal lymph node staging of non-small-cell lung cancer: A prospective comparison of computed tomography and positron emission tomography. J Thorac Cardiovasc Surg. 1996;111(3):642–8.
Vansteenkiste JF, Stroobants SG, De Leyn PR, Dupont PJ, Bogaert J, Maes A, et al. Lymph node staging in non small-cell lung cancer with FDG-PET scan: a prospective study on 690 lymphnode stations from 68 patients. J Clin Oncol. 1998;16:2142–9.
Bryant AS, Cerfolio RJ, Klemm KM, Ojha B. Maximum standard uptake value of mediastinal lymph nodes on integrated FDG-PET-CT predicts pathology in patients with non-small cell lung cancer. Ann Thorac Surg. 2006;82:417–23.
Kumar A, Dutta R, Kannan U, Kumar R, Khilnani GC, Gupta SD. Evaluation of mediastinal lymph nodes using 18 F-FDG PET-CT scan and its histopathologic correlation. Ann Thorac Med. 2011;6:11–6.
Boiselle PM, Patz EF, Vining DJ, Weissleder R, Shepard JA, McLoud TC. Imaging of mediastinal lymph nodes: CT, MR, and FDG PET. Radiographics. 1998;18:1061–9.
Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):178S–201S.
Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ, Han J, et al. Non-small cell lung cancer: Prospective comparison of integrated FDG PET/CTand CT alone for preoperative staging. Radiology. 2005;236:1011–9.
Kim SK, Shin JE, Lee JH. Peripheral tuberculous lymphadenitis masquerading as metastatic gastric carcinoma on F-18 FDG dual time point PET/CT. Nucl Med Mol Imaging. 2012;46:316–7.
Kim YK, Lee KS, Kim BT, Choi JY, Kim H, Kwon OJ, et al. Mediastinal nodal staging of non small cell lung cancer using integrated FDG PET/CT in a tuberculosis-endemic country. Cancer. 2007;109:1068–77.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Budiawan, H., Cheon, G.J., Im, HJ. et al. Heterogeneity Analysis of 18F-FDG Uptake in Differentiating Between Metastatic and Inflammatory Lymph Nodes in Adenocarcinoma of the Lung: Comparison with Other Parameters and its Application in a Clinical Setting. Nucl Med Mol Imaging 47, 232–241 (2013). https://doi.org/10.1007/s13139-013-0216-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-013-0216-6