Abstract
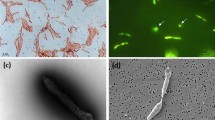
Dimethylsulfoniopropionate (DMSP) is mainly produced by marine phytoplankton as an osmolyte, antioxidant, predator deterrent, or cryoprotectant. DMSP is also an important carbon and sulfur source for marine bacteria. Bacteria may metabolize DMSP via the demethylation pathway involving the DMSP demethylase gene (dmdA) or the cleavage pathway involving several different DMSP lyase genes. Most DMSP released into seawater is degraded by bacteria via demethylation. To test a hypothesis that the high gene frequency of dmdA among major marine taxa results in part from horizontal gene transfer (HGT) events, a total of thirty-one bacterial strains were isolated from Arctic Kongsfjorden seawater in this study. Analysis of 16S rRNA gene sequences showed that, except for strains BSw22118, BSw22131 and BSw22132 belonging to the genera Colwellia, Pseudomonas and Glaciecola, respectively, all bacteria fell into the genus Pseudoalteromonas. DmdA genes were detected in five distantly related bacterial strains, including four Arctic strains (Pseudoalteromonas sp. BSw22112, Colwellia sp. BSw22118, Pseudomonas sp. BSw22131 and Glaciecola sp. BSw22132) and one Antarctic strain (Roseicitreum antarcticum ZS2-28). Their dmdA genes showed significant similarities (97.7%–98.3%) to that of Ruegeria pomeroyi DSS-3, which was originally isolated from temperate coastal seawater. In addition, the sequence of the gene transfer agent (GTA) capsid protein gene (g5) detected in Antarctic strain ZS2-28 exhibited a genetically closely related to that of Ruegeria pomeroyi DSS-3. Among the five tested strains, only Pseudomonas sp. BSw22131 could grow using DMSP as the sole carbon source. The results of this study support the hypothesis of HGT for dmdA among taxonomically heterogeneous bacterioplankton, and suggest a wide distribution of functional gene (i.e., dmdA) in global marine environments.
Similar content being viewed by others
References
Andreae M O. 1990. Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Marine Chemistry, 30: 1–29, doi: 10.1016/0304-4203(90)90059-L
Bürgermeister S, Georgii H W, Zimmermann R L, et al. 1990. On the biogenic origin of dimethylsulfide: relation between chlorophyll, ATP, organismic DMSP, phytoplankton species, and DMS distribution in Atlantic surface water and atmosphere. Journal of Geophysical Research: Atmosphere, 95(D12): 20607–20615, doi: 10.1029/JD095iD12p20607
Biers E J, Wang Kui, Pennington C, et al. 2008. Occurrence and expression of gene transfer agent genes in marine bacterioplank-ton. Applied and Environmental Microbiology, 74(10): 2933–2939, doi: 10.1128/AEM.02129-07
Bullock H A, Luo Haiwei, Whitman W B. 2017. Evolution of dimethyl-sulfoniopropionate metabolism in marine phytoplankton and bacteria. Frontiers in Microbiology, 8: 637, doi: 10.3389/ fmicb.2017.00637
Charlson R J, Lovelock J E, Andreae M O, et al. 1987. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature, 326(6114): 655–661, doi: 10.1038/326655a0
Cui Yingshun, Suzuki S, Omori Y, et al. 2015. Abundance and distribution of dimethylsulfoniopropionate degradation genes and the corresponding bacterial community structure at dimethyl sulfide hot spots in the tropical and subtropical Pacific Ocean. Applied and Environmental Microbiology, 81(12): 4184–4194, doi: 10.1128/AEM.03873-14
Curson A R J, Todd J D, Sullivan M J, et al. 2011. Catabolism of dimethylsulphoniopropionate: microorganisms, enzymes and genes. Nature Reviews Microbiology, 9(12): 849–859, doi: 10.1038/nrmicro2653
Fu Yunyun, MacLeod D M, Rivkin R B, et al. 2010. High diversity of Rhodobacterales in the subarctic North Atlantic Ocean and gene transfer agent protein expression in isolated strains. Aquatic Microbial Ecology, 59(3): 283–293, doi: 10.3354/ ame01398
Fuhrman J A, Lee S H, Masuchi Y, et al. 1994. Characterization of marine prokaryotic communities via DNA and RNA. Microbial Ecology, 28(2): 133–145, doi: 10.1007/BF00166801
Gonzalez J M, Covert J S, Whitman W B, et al. 2003. Silicibacter pomeroyi sp. nov. and Roseovarius nubinhibens sp. nov., dimethylsulfoniopropionate-demethylating bacteria from marine environments. International Journal of Systematic and Evolutionary Microbiology, 53(5): 1261–1269, doi: 10.1099/ ijs.0.02491-0
Gonzalez J M, Kiene R P, Moran M A. 1999. Transformation of sulfur compounds by an abundant lineage of marine bacteria in the a-subclass of the class Proteobacteria. Applied and Environmental Microbiology, 65(9): 3810–3819
Guillard R R L, Ryther J H. 1962. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology, 8(2): 229–239, doi: 10.1139/m62-029
Herlemann D P R, Woelk J, Labrenz M, et al. 2014. Diversity and abundance of “Pelagibacterales” (SAR11) in the Baltic Sea salinity gradient. Systematic and Applied Microbiology, 37(8): 601–604, doi: 10.1016/j.syapm.2014.09.002
Hollibaugh J T, Bano N, Ducklow H W. 2002. Widespread distribution in polar oceans of a 16S rRNA gene sequence with affinity to Nitrosospira-like ammonia-oxidizing bacteria. Applied and Environmental Microbiology, 68(3): 1478–1484, doi: 10.1128/ AEM.68.3.1478-1484.2002
Howard E C, Henriksen J R, Buchan A, et al. 2006. Bacterial taxa that limit sulfur flux from the ocean. Science, 314(5799): 649–652, doi: 10.1126/science.ll30657
Howard E C, Sun Shulei, Biers E J, et al. 2008. Abundant and diverse bacteria involved in DMSP degradation in marine surface waters. Environmental Microbiology, 10(9): 2397–2410, doi: 10.1111/J.1462-2920.2008.01665.X
Howard E C, Sun Shulei, Reisch C R, et al. 2011. Changes in dimethyl-sulfoniopropionate demethylase gene assemblages in response to an induced phytoplankton bloom. Applied and Environmental Microbiology, 77(2): 524–531, doi: 10.1128/ AEM.01457-10
Huber T, Faulkner G, Hugenholtz P. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics, 20(14): 2317–2319, doi: 10.1093/bioinformat-ics/bth226
Johnston A W B, Green R T, Todd J D. 2016. Enzymatic breakage of dimethylsulfoniopropionate—a signature molecule for life at sea. Current Opinion in Chemical Biology, 31: 58–65, doi: 10.1016/j.cbpa.2016.01.011
Johnston A W B, Todd J D, Sun Lei, et al. 2008. Molecular diversity of bacterial production of the climate-changing gas, dimethyl sulphide, a molecule that impinges on local and global symbioses. Journal of Experimental Botany, 59(5): 1059–1067, doi: 10.1093/jxb/erm264
Karsten U, Kiick K, Vogt C, et al. 1996. Dimethylsulfoniopropionate production in phototrophic organisms and its physiological functions as a cryoprotectant. In: Kiene R P, Visscher P T, Keller M D, et al., eds. Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. New York: Plenum Press, 143–153
Keller M D. 1989. Dimethyl sulfide production and marine phytoplankton: the importance of species composition and cell size. Biological Oceanography, 6(5-6): 375–382, doi: 10.1080/01965581. 1988.10749540
Kiene R P. 1990. Dimethyl sulfide production from dimethylsulfoniopropionate in coastal seawater samples and bacterial cultures. Applied and Environmental Microbiology, 56(11): 3292–3297
Kiene R P, Linn L J. 2000. Distribution and turnover of dissolved DMSP and its relationship with bacterial production and dimethyl-sulfide in the Gulf of Mexico. Limnology and Oceanography, 45(4): 849–861, doi: 10.4319/lo.2000.45.4.0849
Kiene R P, Linn L J, Bruton J A. 2000. New and important roles for DMSP in marine microbial communities. Journal of Sea Research, 43(3-4): 209–224, doi: 10.1016/S1385-1101(00)00023-X
Kiene R P, Linn L J, J, et al. 1999. Dimethylsulfoniopropionate and methanethiol are important precursors of methionine and protein-sulfur in marine bacterioplankton. Applied and Environmental Microbiology, 65(10): 4549–4558
Kirst G O, Thiel C, Wolff H, et al. 1990. Dimethylsulfoniopropionate (DMSP) in icealgae and its possible biological role. Marine Chemistry, 35(1-4): 381–388, doi: 10.1016/S0304-4203 (09)90030-5
Lang A S, Beatty J T. 2007. Importance of widespread gene transfer agent genes in a-proteobacteria. Trends in Microbiology, 15(2): 54–62, doi: 10.1016/j.tim.2006.12.001
Lang A S, Zhaxybayeva O, Beatty J T. 2012. Gene transfer agents: phage-like elements of genetic exchange. Nature Reviews Microbiology, 10(7): 472–482, doi: 10.1038/nrmicro2802
Marrs B. 1974. Genetic recombination in Rhodopseudomonas capsulata. Proceedings of the National Academy of Sciences of the United States of America, 71(3): 971–973, doi: 10.1073/pnas. 71.3.971
McDaniel L D, Young E, Delaney J, et al. 2010. High frequency of horizontal gene transfer in the oceans. Science, 330(6000): 50, doi: 10.1126/science.ll92243
Moran M A, Reisch C R, Kiene R P, et al. 2012. Genomic insights into bacterial DMSP transformations. Annual Review of Marine Science, 4: 523–542, doi: 10.1146/annurev-marine-120710-100827
Pawlowski J, Fahrni J, Lecroq B, et al. 2007. Bipolar gene flow in deep-sea benthic foraminifera. Molecular Ecology, 16(19): 4089–4096, doi: 10.1111/J.1365-294X.2007.03465.X
Pommier T, Pinhassi J, Hagstrom A. 2005. Biogeographic analysis of ribosomal RNA clusters from marine bacterioplankton. Aquatic Microbial Ecology, 41(1): 79–89, doi: 10.3354/ame041079
Reisch C R, Moran M A, Whitman W B. 2008. Dimethylsulfoniopropi-onate-dependent demethylase (DmdA) from Pelagibacter ubi-que and Silicibacter pomeroyi. Journal of Bacteriology, 190(24): 8018–8024, doi: 10.1128/JB.00770-08
Reisch C R, Stoudemayer M J, Varaljay V A, et al. 2011. Novel pathway for assimilation of dimethylsulphoniopropionate widespread in marine bacteria. Nature, 473(7346): 208–211, doi: 10.1038/naturel0078
Ripp S, Miller R V. 1995. Effects of suspended particulates on the frequency of transduction among Pseudomonas aeruginosa in a freshwater environment. Applied and Environmental Microbiology, 61(4): 1214–1219
Salgado P, Kiene R, Wiebe W, et al. 2014. Salinity as a regulator of DMSP degradation in Ruegeria pomeroyi DSS-3. Journal of Microbiology, 52(11): 948–954, doi: 10.1007/sl2275-014-4409-1
Sambrook J, Russell D W. 2001. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press
Schwartz S E, Andreae M O. 1996. Uncertainty in climate change caused by aerosols. Science, 272(5265). 1121. doi: 10.1126/sci-ence.272.5265.1121
Shaw G E. 1983. Bio-controlled thermostasis involving the sulfur cycle. Climatic Change, 5(3): 297–303, doi: 10.1007/BF02423524
Solioz M, Marrs B. 1977. The gene transfer agent of Rhodopseudomonas capsulata: purification and characterization of its nucleic acid. Archives of Biochemistry and Biophysics, 181(1): 300–307, doi: 10.1016/0003-9861(77)90508-2
Sun Jing, Todd J D, Thrash J C, et al. 2016. The abundant marine bacterium Pelagibacter simultaneously catabolizes dimethylsulfoniopropionate to the gases dimethyl sulfide and methanethiol. Nature Microbiology, 1(8). 16065. doi: 10.1038/nmicrobiol.2016.65
Sunda W, Kieber D J, Kiene R P, et al. 2002. An antioxidant function for DMSP and DMS in marine algae. Nature, 418(6895): 317–320, doi: 10.1038/nature00851
Tamura K, Peterson D, Peterson N, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10): 2731–2739, doi: 10.1093/mol-bev/msrl21
Thompson J D, Higgins D G, Gibson T J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22(22): 4673–4680, doi: 10.1093/nar/22.22.4673
Turner S M, Nightingale P D, Broadgate W, et al. 1995. The distribution of dimethyl sulphide and dimethylsulphoniopropionate in Antarctic waters and sea ice. Deep Sea Research Part II: Topical Studies in Oceanography, 42(4-5): 1059–1080, doi: 10.1016/ 0967-0645(95)00066-y
Vettori C, Stotzky G, Yoder M, et al. 1999. Interaction between bacteriophage PBS1 and clay minerals and transduction of Bacillus subtilis by clay-phage complexes. Environmental Microbiology, 1(4): 347–355, doi: 10.1046/J.1462-2920.1999.00044.X
Visscher P T, Diaz M R, Taylor B F. 1992. Enumeration of bacteria which cleave or demethylate dimethylsulfoniopropionate in the Caribbean Sea. Marine Ecology Progress Series, 89: 293–296, doi: 10.3354/meps089293
Weinbauer M G. 2004. Ecology of prokaryotic viruses. FEMS Microbiology Reviews, 28(2): 127–181, doi: 10.1016/j.femsre.2003. 08.001
Wolfe G V, Steinke M, Kirst G O.1997. Grazing-activated chemical defence in a unicellular marine alga. Nature, 387(6636): 894–897, doi: 10.1038/43168
Yen H C, Hu N T, Marrs B L. 1979. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomo-nas capsulata. Journal of Molecular Biology, 131(2): 157–168, doi: 10.1016/0022-2836(79)90071-8
Yu Yong, Yan Shulin, Li Huirong, et al. 2011. Roseicitreum antarctic-um gen. nov., sp. nov., an aerobic bacteriochlorophyll a-con-taining alphaproteobacterium isolated from Antarctic sandy in-tertidal sediment. International Journal of Systematic and Evolutionary Microbiology, 61(9): 2173–2179, doi: 10.1099/ijs.0.024885-0
Zeng Yinxin, Liu Wenqi, Li Huirong, et al. 2007. Effect of restriction endonucleases on assessment of biodiversity of cultivable polar marine planktonic bacteria by amplified ribosomal DNA restriction analysis. Extremophiles, 11(5): 685–692, doi: 10.1007/S00792-007-0086-X
Zeng Yinxin, Qiao Zongyun, Yu Yong, et al. 2016. Diversity of bacterial dimethylsulfoniopropionate degradation genes in surface seawater of Arctic Kongsfjorden. Scientific Reports, 6. 33031. doi: 10.1038/srep33031
Zhao Yanlin, Wang Kui, Budinoff C, et al. 2008. Gene transfer agent (GTA) genes reveal diverse and dynamic Roseobacter and Rhodobacter populations in the Chesapeake bay. The ISME Journal, 3(3): 364–373, doi: 10.1038/ismej.2008.115
Acknowledgements
I appreciate the assistance of the Chinese Arctic and Antarctic Administration (CAA) who organized the Chinese Arctic Yellow River Station Expedition in 2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: The National Natural Science Foundation of China under contract No. 41476171; the Chinese Polar Environment Comprehensive Investigation and Assessment Program under contract No. CHINARE04-01.
Rights and permissions
About this article
Cite this article
Zeng, Y. Phylogenetic diversity of dimethylsulfoniopropionatedependent demethylase gene dmdA in distantly related bacteria isolated from Arctic and Antarctic marine environments. Acta Oceanol. Sin. 38, 64–71 (2019). https://doi.org/10.1007/s13131-019-1393-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-019-1393-7