Abstract
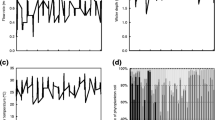
Research of interaction mechanism between Chlorella vulgaris and two bacterial strains (Z-QD08 and Z-QS01) were conducted under laboratory conditions. Growth rates of bacteria and C. vulgaris were tested under co-culture conditions to evaluate the effects of concentrations of C. vulgaris and bacteria on their interactions. To test whether the availability of inorganic nutrients, vitamins and trace metals affects the interactions between C. vulgaris and bacteria, experiments were performed with or without the culture medium filtrate of C. vulgaris or bacteria. The results showed that the growth of C. vulgaris was promoted at low concentrations of bacteria (5×106 cells/ml), and expressed a positive correlation with the bacteria density, whereas opposite trend was observed for treatments with high bacteria density (10×106 cells/ml and 20×106 cells/ml). The growth rate of bacteria decreased with the increasing concentrations of C. vulgaris. The growth of bacteria Z-QD08 was inhibited by C. vulgaris through interference competition, while the mechanism for interaction between bacteria Z-QS01 and C. vulgaris was resource competition. The influence of cell density on the interaction between microalgae and bacteria was also discussed. These experiments confirm some elements of published theory on interactions between heterotrophic bacteria and microalgae and suggest that heterotrophic bacteria play an important role in the development of blooms in natural waters.
Similar content being viewed by others
References
Chen Wenming, Sheu F S, Sheu S Y. 2012. Aquimarina salinaria sp. nov., a novel algicidal bacterium isolated from a saltpan. Archives of Microbiology, 194: 103–112
Croft MT, Lawrence A D, Raux-Deery E, et al. 2005. Algae acquire vitamin B12 through a symbiotic relation ship with bacteria. Nature, 438: 90–93
Danger M, Oumarou C, Benest D, et al. 2007. Bacteria can control stoichiometry and nutrient limitation of phytoplankton. Functional Ecology, 21: 202–210
Daufresne T, Loreau M. 2001. Plant-herbivore interactions and ecological stoichiometry: when do herbivores determine plant nutrient limitation?. Ecology Letters, 4: 196–206
DellaGreca M, Zarrelli A, Fergola P, et al. 2010. Fatty acids released by Chlorella vulgaris and their role in interference with Pseudokirchneriella subcapitata: experiments and modeling. Journal of Chemical Ecology, 36: 339–349
Du Jingjing, Zhao Guiying, Wang Fangyuan, et al. 2013. Growth stimulation of Microcystis aeruginosa by a bacterium from hypereutrophic water (Taihu Lake, China). Aquatic Ecology, 47: 303–313
Fukami, K, Nishijima T, Ishida Y. 1997. Stimulative and inhibitory effects of bacteria on the growth of microalgae. Hydrobiologia, 358: 185–191
Furusawa G, Yoshikawa T, Yasuda A, et al. 2003. Algicidal activity and gliding motility of Saprospira sp. SS98-5. Canadian Journal of Microbiology, 49: 92–100
Gonzalez L E, Bashan Y. 2000. Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Applied and Environmental Microbiology, 66(4): 1527–1531
Grover J P. 2000. Resource competition and community structure in aquatic microorganisms: experimental studies of algae and bacteria along a gradient of organic carbon to inorganic phosphorus supply. Journal of Plankton Research, 22(8): 1591–1610
Grossart H P, Simon M. 2007. Interactions of planktonic algae and bacteria: effects on algal growth and organic matter dynamics. AquaticMicrobial Ecology, 47: 163–176
Guillard R R L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH, eds. Culture of Marine Animals. New York: PlenumPress, 26–60
Lekunberri I, Lefort T, Romera-Castillo C, et al. 2012. Relationship between induced phytoplankton blooms and the structure and dynamics of the free-living heterotrophic bacterial community. Marine Ecological Progress Series, 448: 23–37
Lovejoy C, Bowman J P, Hallegraeff G M. 1998. Algicidal effects of a novel marine Pseudoalteromonas isolate (Class Proteobacteria, Gamma Subdivision) on harmful algal bloom species of the genera Chattonella, Gymnodinium, and Heterosigma. Applied and Environmental Microbiology, 64: 2806–2813
Mayali X, Azam F. 2004. Algicidal Bacteria in the Sea and their Impact on Algal Blooms. Journal of Eukaryotic Microbiology, 51(2): 139–144
Mouget J L, Dakhama A, Lavoie M C, et al. 1995. Algal growth enhancement by bacteria: Is consumption of photosynthetic oxygen involved?. Microbiology Ecology, 18: 35–44
Nakashima T J, Miyazak Y, Matsuyama Y, et al. 2006. Producing mechanism of an algicidal compound against red tide phytoplankton in a marine bacterium γ-proteobacterium. Applied Microbiology and Biotechnology, 73: 684–690
Park Y, Je K W, Lee K Y, et al. 2008. Growth promotion of Chlorella ellipsoidea by co-inoculation with Brevundimonas sp. isolated from the microalga. Hydrobiologia, 598: 219–228
Pinhassi J, Sala M M, Havskum H, et al. 2004. Changes in bacterioplankton composition under different phytoplankton regimens. Applied and Environmental Microbiology, 70(11): 6753–6766
Regunathan C, Wesley S G. 2004. Control of Vibrio spp. in shrimp hatcheries using the green algae Tetraselmis suecica. Asian Fisheries Science, 17: 147–158
Ribalet F, Intertaglia L, Lebaron P, et al. 2008. Differential effect of three polyunsaturated aldehydes on marine bacterial isolates. Aquatic Toxicology, 86(2): 249–255
Rooney-Varga J N, Giewat M W, Savin M C, et al. 2005. Links between phytoplankton and bacterial community dynamics in a coastal marine environment. Microbial Ecology, 49: 163–175
Sakata T, Yoshikawa T, Nishitarumizu S. 2011. Algicidal activity and identification of an algicidal substance produced by marine Pseudomonas sp. C55a-2. Fish Science, 77: 397–402
Spoerner M, Wichard T, Bachhuber T, et al. 2012. Growth and Thallus Morphogenesis of Ulva mutabilis (Chlorophyta) depends on a combination of two bacterial species excreting regulatory factors. Journal of Phycology, 48: 1433–1447
Spolaore P, Joannis-Cassan C, Duran E, et al. 2006. Commercial applications of microalgae. Journal of Bioscience and Bioengineering, 101(2): 87–96
Trabelsia A, Rassoulzadegana F. 2011. Inorganic nutrient control of dissolved organic carbon (DOC) dynamics in NW Mediterranean waters: An experimental approach. Marine Biology Research, 7: 667–676
Vatsa P, Sanchez L, Clement C, et al. 2010. Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. International Journal of Molecular Sciences, 11: 5095–5108
Watanabe K J, Takihana N, Aoyagi H, et al. 2005. Symbiotic association in Chlorella culture. Microbiology Ecology, 51: 187–196
Zhao Su, Pan Weibin, Ma Chao. 2012. Stimulation and inhibition effects of algae-lytic products from Bacillus cereus strain L7 on Anabaena flosaquae. Journal of Applied Phycology, 24: 1015–1021
Zhou Wenli, Qiao Xiuting, Sun Jingfeng, et al. 2011. Ecological effect of Z-QS01 Strain on Chlorella vulgaris and its response to UV-B radiation stress. Procedia Environmental Sciences, 11: 741–748
Zoppini A, Puddu A, Fazi S, et al. 2005. Extracellular enzyme activity and dynamics of bacterial community in mucilaginous aggregates of the northern Adriatic Sea. Science of The Total Environment, 353: 270–286
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Key Projects of Applied Basic and Frontier Technology Research of Tianjin under contract No. 13JCZDJC29300; scientific research plan program of Tianjin Agricultural University under contract No. 2009D005; the National Natural Science Foundation of China under contract No. 31200400.
Rights and permissions
About this article
Cite this article
Qu, L., Wang, R., Zhao, P. et al. Interaction between Chlorella vulgaris and bacteria: interference and resource competition. Acta Oceanol. Sin. 33, 135–140 (2014). https://doi.org/10.1007/s13131-014-0432-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-014-0432-7