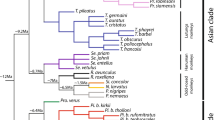
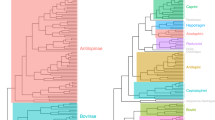
Abstract
Convergent evolution is often reported in the mammalian order Carnivora. Their adaptations to particularly demanding feeding habits such as hypercarnivory and durophagy (consumption of tough food) appear to favour morphological similarities between distantly related species, especially in the skull. However, phylogenetic effect in phenotypic data might obscure such a pattern. We first validated the hypotheses that extant hypercarnivorous and durophagous large carnivorans converge in mandibular shape and form (size and shape). Hypercarnivores generally exhibit smaller volumes of the multidimensional shape and form space than their sister taxa, but this pattern is significantly different from random expectation only when hunting behaviour categorisations are taken into account. Durophages share areas of the morphospace, but this seems to be due to factors of contingency. Carnivorans that hunt in pack exhibit incomplete convergence while even stronger similarities occur in the mandible shape of solitary hunters due to the high functional demands in killing the prey. We identified a stronger phylogenetic signal in mandibular shape than in size. The quantification of evolutionary rates of changes suggests that mandible shape of solitary hunters evolved slowly when compared with other carnivorans. These results consistently indicate that the need for a strong bite force and robust mandible override sheer phylogenetic effect in solitary hunters.


Similar content being viewed by others
References
Adams, D. C. (2014a). A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Systematic Biology, 63, 685–697.
Adams, D. C. (2014b). Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Systematic Biology, 63, 166–177.
Adams, D. C., & Collyer, M. L. (2015). Permutation tests for phylogenetic comparative analyses of high-dimensional shape data: what you shuffle matters. Evolution, 69, 823–829.
Adams, D. C., & Otárola-Castillo, E. (2013). Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution, 4, 393–399.
Adams, D. C., Cardini, A., Monteiro, L. R., O'Higgins, P., & Rohlf, F. J. (2011). Morphometrics and phylogenetics: principal components of shape from cranial modules are neither appropriate nor effective cladistic characters. Journal of Human Evolution, 60, 240–243.
Adams, D. C., Rohlf, D. C., & Slice, D. E. (2013). A field comes of age: geometric morphometrics in the 21st century. Hystrix, the Italian Journal of Mammalogy, 24, 7–14.
Allen, M. E., Oftedal, O. T., & Baer, D. J. (1996). The feeding and nutrition of carnivores. In D. G. Kleiman, M. E. Allen, K. V. Thompson, & S. Lumpkin (Eds.), Wild mammals in captivity. Principles and Techniques (pp. 139–147). Chicago: University of Chicago Press.
Arnold, C., Matthews, L. J., & Nunn, C. L. (2010). The 10k trees Website: a new online resource for primate phylogeny. Evolutionary Anthropology, 19, 114–118.
Bennett, C. V., & Goswami, A. (2013). Statistical support for the hypothesis of developmental constraint in marsupial skull evolution. BMC Biology, 11(1), 52.
Biknevicius, A. R., & Van Valkenburgh, B. (1996). Design for killing: craniodental adaptations of mammalian predators. In J. L. Gittleman (Ed.), Carnivore behavior, ecology, and evolution, Vol. 2 (pp. 393–428). Ithaca, New York: Cornell University Press.
Blomberg, S. P., Garland, T., Jr., & Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57, 717–745.
Bookstein, F.L. (1989). Size and shape: a comment on semantics. Systematic Zoology, 38, 173–180.
Carbone, C., Georgina, M. M., Roberts, S. C., & Macdonald, D. W. (1999). Energetic constraints on the diet of terrestrial carnivores. Nature, 402, 286–288.
Carbone, C., Teacher, A., & Rowcliffe, J. M. (2007). The cost of carnivory. PLoS Biology, 5, e22.
Clauss, M., Kleffner, H., & Kienzle, E. (2010). Carnivorous mammals: nutrient digestibility and energy evaluation. Zoo Biology, 29, 687–704.
Creel, S., & Creel, N.M. (2002). The African wild dog: behavior, ecology, and conservation. Princeton University Press.
Davies, J. T., Meiri, S., Barraclough, T. G., & Gittleman, J. L. (2007). Species co‐existence and character divergence across carnivores. Ecology Letters, 10, 146–152.
Echarri, S., & Prevosti, F. J. (2015). Differences in mandibular disparity between extant and extinct species of metatherian and placental carnivore clades. Lethaia, 48, 196–204.
Ewer, R. F. (1973). The carnivores. New York: Cornell University Press.
Figueirido, B., Serrano-Alarcón, F. J., Slater, G. J., & Palmqvist, P. (2010). Shape at the cross-roads: homoplasy and history in the evolution of the carnivoran skull towards herbivory. Journal of Evolutionary Biology, 23, 2579–2594.
Figueirido, B., MacLeod, N., Krieger, J., De Renzi, M., Pérez-Claros, J. A., & Palmqvist, P. (2011). Constraint and adaptation in the evolution of carnivoran skull shape. Paleobiology, 37, 490–518.
Figueirido, B., Tseng, Z. J., & Martin-Serra, A. (2013). Skull shape evolution in durophagous carnivorans. Evolution, 67, 1975–1993.
Friscia, A. R., Van Valkenburgh, B., & Biknevicius, A. R. (2007). An ecomorphological analysis of extant small carnivorans. Journal of Zoology (London), 272, 82–100.
Futuyma, D. J. (2010). Evolutionary constraint and ecological consequences. Evolution, 64, 1865–1884.
Goodall, C. (1991). Procrustes methods in the statistical analysis of shape. Journal of the Royal Statistical Society. Series B (Methodological), 285–339.
Goswami, A. (2006). Morphological integration in the carnivoran skull. Evolution, 60, 122–136.
Goswami, A. (2010). Introduction to carnivoran evolution. In A. Goswami & A. Friscia (Eds.), Carnivoran evolution: new views on phylogeny, form, and function (pp. 1–24). Cambridge: Cambridge University Press.
Harmon, L. J., Schulte, J. A., Losos, J. B., & Larson, A. (2003). Tempo and mode of evolutionary radiation in iguanian lizards. Science, 301, 961–964.
Holliday, J. A., & Steppan, S. J. (2004). Evolution of hypercarnivory: the effect of specialization on morphological and taxonomic diversity. Paleobiology, 30, 108–128.
Johnson, K. G., Schaller, G. B., & Jinchu, H. (1988). Comparative behavior of red and giant pandas in the Wolong Reserve, China. Journal of Mammalogy, 69, 552–564.
Kruuk, H. (1972). The spotted hyena (a study of predation and social behaviour). Chicago and London: The University of Chicago Press.
Lofroth, E. C., Krebs, J. A., Harrower, W. L., & Lewis, D. (2007). Food habits of wolverine Gulo gulo in montane ecosystems of British Columbia, Canada. Wildlife Biology, 13, 31–37.
Mech, D. (1980). The wolf. The ecology and behaviour of an endangered species. Minneapolis, London: University of Minnesota Press.
Meiri, S., Guy, D., Dayan, T., & Simberloff, D. (2009). Global change and carnivore body size: data are stasis. Global Ecology and Biogeography, 18, 240–247.
Meloro, C. (2011a). Feeding habits of Plio–Pleistocene large carnivores as revealed by their mandibular geometry. Journal of Vertebrate Paleontology, 31, 428–446.
Meloro, C. (2011b). Morphological disparity in Plio–Pleistocene large carnivore guilds from Italian peninsula. Acta Palaeontologica Polonica, 56, 33–44.
Meloro, C. (2012). Mandibular shape correlates of tooth fracture in extant Carnivora: implications to inferring feeding behaviour of Pleistocene predators. Biological Journal of the Linnean Society, 106, 70–80.
Meloro, C., & O’Higgins, P. (2011). Ecological adaptations of mandibular form in fissiped Carnivora. Journal of Mammalian Evolution, 18, 185–200.
Meloro, C., & Raia, P. (2010). Cats and dogs down the tree: the tempo and mode of evolution in the lower carnassial of fossil and living Carnivora. Evolutionary Biology, 37, 177–186.
Meloro, C., Raia, P., Piras, P., Barbera, C., & O’Higgins, P. (2008). The shape of the mandibular corpus in large fissiped carnivores: allometry, function and phylogeny. Zoological Journal of the Linnean Society, 154, 832–845.
Meloro, C., Raia, P., Carotenuto, F., & Cobb, S. (2011). Phylogenetic signal, function and integration in the subunits of the carnivoran mandible. Evolutionary Biology, 38, 465–475.
Meloro, C., Cáceres, N.C., Carotenuto, F., Sponchiado, J., Melo, G.L., Passaro, F., & Raia P. (2015). Chewing on the trees: constraints and adaptation in the evolution of the 1 primate mandible. Evolution (in press).
O'Higgins, P., & Jones, N. (1998). Facial growth in Cercocebus torquatus: an application of three dimensional geometric morphometric techniques to the study of morphological variation. Journal of Anatomy, 193, 251–272.
Prevosti, F. J., Turazzini, G. F., Ercoli, M. D., & Hingst–Zaher, E. (2012). Mandible shape in marsupial and placental carnivorous mammals: a morphological comparative study using geometric morphometrics. Zoological Journal of the Linnean Society, 164, 836–855.
Raia, P. (2004). Morphological correlates of tough food consumption in carnivores. Italian Journal of Zoology, 71, 45–50.
Raia, P., Carotenuto, F., Meloro, C., Piras, P., & Pushkina, D. (2010). The shape of contention: adaptation, history, and contingency in ungulate mandibles. Evolution, 64, 1489–1503.
Rohlf, F. J., & Slice, D. E. (1990). Extensions of the procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39, 40–59.
Schaller, G. B. (1972). The Serengeti lion (a study of predator–prey relations). Chicago and London: The University of Chicago Press.
Slater, G. J., Figueirido, B., Louis, L., Yang, P., & Van Valkenburgh, B. (2010). Biomechanical consequences of rapid evolution in the polar bear lineage. PloS One, 5(11), e13870.
Stayton, C. T. (2006). Testing hypotheses of convergence with multivariate data: morphological and functional convergence among herbivorous lizards. Evolution, 60, 824–841.
Van Valkenburgh, B. (1985). Locomotor diversity between past and present guilds of large predatory mammals. Paleobiology, 11, 406–428.
Van Valkenburgh, B. (1988). Trophic diversity in past and present guilds of large predatory mammals. Paleobiology, 14, 155–173.
Van Valkenburgh, B. (1989). Carnivore dental adaptations and diet: a study of trophic diversity within guilds. In J. L. Gittleman (Ed.), Carnivore behavior, ecology, and evolution, volume 1 (pp. 410–436). Ithaca: Cornell University Press.
Van Valkenburgh, B. (1991). Iterative evolution of hypercarnivory in canids (Mammalia: Carnivora): evolutionary interactions among sympatric predators. Paleobiology, 17, 340–362.
Van Valkenburgh, B. (1996). Feeding behaviour in free-ranging, large African carnivores. Journal of Mammalogy, 77, 240–254.
Van Valkenburgh, B. (1999). Major patterns in the history of carnivorous mammals. Annual Review Earth Planetary Science, 27, 463–493.
Van Valkenburgh, B. (2007). Déjà vu: the evolution of feeding morphologies in the Carnivora. Integrative Comparative Biology, 47, 147–163.
Wei, F., Feng, Z., Wang, Z., Zhou, A., & Hu, J. (1999). Use of the nutrients in bamboo by the red panda (Ailurus fulgens). Journal of Zoology (London), 248, 535–541.
Werdelin, L. (1989). Constraint and adaptation in the bone-cracking canid Osteoborus (Mammalia: Canidae). Paleobiology, 15, 387–401.
Acknowledgements
We are grateful to F. Carotenuto and N. Cacéres for their comments and support. F. Prevosti, one anonymous reviewer and A. Wanninger provided considerable support to improve the quality of this manuscript. Carlo Meloro was supported by TEMASAV/DOTTORI DI RICERCA-ESPERTI/26.
Conflict of interest
The authors declare no conflict of interests in this publication and confirm that it is an original work that did not involved humans or animals. In addition, all the authors confirm that they were actively involved in the realisation of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOCX 1176 kb)
Rights and permissions
About this article
Cite this article
Meloro, C., Clauss, M. & Raia, P. Ecomorphology of Carnivora challenges convergent evolution. Org Divers Evol 15, 711–720 (2015). https://doi.org/10.1007/s13127-015-0227-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-015-0227-5