Abstract
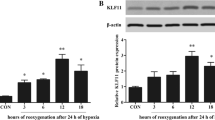
A series of protective responses could be evoked to achieve compensatory adaptation once cardiomyocytes are subjected to chronic hypoxia. MLK3/JNK/c-jun signaling pathway was previously demonstrated to be involved in this process. In the present study, we aim to further examine the performance of MLK3 in hypoxic H9C2 cells and potential mechanism. Myocardial samples of patients with congenital heart disease (CHD) were collected. H9C2 cells were cultured in hypoxic conditions for various durations. MLK3 was silenced by transfection of shRNA to evaluate its role in cell viability. We found expression of MLK3 protein was lower in patients with cyanotic CHD. In hypoxic H9C2 cells, its expression was gradually decreased in a time-dependent manner. However, there was no significant difference about expression of MLK3 mRNA. According to the results of MTT, LDH, and TUNEL, faster cell growth curve, lower death rate, and less apoptotic cells could be observed in MLK-shRNA group compared with scramble-shRNA group. Silencing of MLK3 significantly reduced expression of cleaved caspase-3, cleaved PARP, Bad, and Bax, together with increased expression of Bcl-2 and ration of Bcl-2/Bax. Both ratio of phospho-JNK/total JNK and ratio of phospho-c-jun/total c-jun were significantly decreased once MLK3 was silenced. At various reoxygenation time, MLK3 shRNA could significantly promote cell survival and decrease cell death according to MTT and LDH. Our results suggested that chronic hypoxia could reduce MLK3 expression in a posttranscriptional regulatory manner. Downregulation of MLK3 protects H9C2 cells from hypoxia-induced apoptosis and H/R injury via blocking the activation of JNK and c-jun.




Similar content being viewed by others
Change history
05 October 2017
Volume 73 issue 3 was published with an incorrect cover date. Correct is August 2017. The Publisher apologizes for this mistake and all related inconveniences caused by this.
References
Abdelli S, Abderrahmani A, Hering BJ, Beckmann JS, Bonny C (2007) The c-Jun N-terminal kinase JNK participates in cytokine- and isolation stress-induced rat pancreatic islet apoptosis. Diabetologia 50:1660–1669
Cao S, Zeng Z, Wang X, Bin J, Xu D, Liao Y (2013) Pravastatin slows the progression of heart failure by inhibiting the c-Jun N-terminal kinase-mediated intrinsic apoptotic signaling pathway. Mol Med Rep 8:1163–1168. doi:10.3892/mmr.2013.1622
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12:861–874
Gu Q, Kong Y, Yu ZB, Bai L, Xiao YB (2011) Hypoxia-induced SOCS3 is limiting STAT3 phosphorylation and NF-kappaB activation in congenital heart disease. Biochimie 93:909–920
Han Q, Yeung SC, Ip MS, Mak JC (2014) Cellular mechanisms in intermittent hypoxia-induced cardiac damage in vivo. J Physiol Biochem 70:201–213
He P, Zhang B, Liu D, Bian X, Li D, Wang Y, Sun G, Zhou G (2016) Hepatitis B virus X protein modulates apoptosis in NRK-52E cells and activates Fas/FasL through the MLK3-MKK7-JNK3 signaling pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 39:1433–1443
He S, Liu P, Jian Z, Li J, Zhu Y, Feng Z, Xiao Y (2013) miR-138 protects cardiomyocytes from hypoxia-induced apoptosis via MLK3/JNK/c-jun pathway. Biochem Biophys Res Commun 441:763–769
Jia W, Jian Z, Li J, Luo L, Zhao L, Zhou Y, Tang F, Xiao Y (2016) Upregulated ATF6 contributes to chronic intermittent hypoxia-afforded protection against myocardial ischemia/reperfusion injury. Int J Mol Med 37:1199–1208
Jian Z, Li JB, Ma RY, Chen L, Wang XF, Xiao YB (2012) Pivotal role of activating transcription factor 6alpha in myocardial adaptation to chronic hypoxia. Int J Biochem Cell Biol 44:972–979
Jian Z, Li JB, Ma RY, Chen L, Zhong QJ, Wang XF, Wang W, Hong Y, Xiao YB (2009) Increase of macrophage migration inhibitory factor (MIF) expression in cardiomyocytes during chronic hypoxia. Clinica chimica acta; international journal of clinical chemistry 405:132–138
Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, Hewett TE, Molkentin JD (2005) Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. J Biol Chem 280:32602–32608
Kim SO, Irwin P, Katz S, Pelech SL (1998) Expression of mitogen-activated protein kinase pathways during postnatal development of rat heart. J Cell Biochem 71:286–301
Kolar F, Ostadal B (2004) Molecular mechanisms of cardiac protection by adaptation to chronic hypoxia. Physiological research / Academia Scientiarum Bohemoslovaca 53(Suppl 1):S3–13
Li JW, He SY, Feng ZZ, Zhao L, Jia WK, Liu P, Zhu Y, Jian Z, Xiao YB (2015) MicroRNA-146b inhibition augments hypoxia-induced cardiomyocyte apoptosis. Mol Med Rep 12:6903–6910
Li X, Liu Y, Ma H, Guan Y, Cao Y, Tian Y, Zhang Y (2016) Enhancement of glucose metabolism via PGC-1alpha participates in the cardioprotection of chronic intermittent hypobaric hypoxia. Front Physiol 7:219
Liang Q, Molkentin JD (2003) Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J Mol Cell Cardiol 35:1385–1394
Ma HJ, Li Q, Ma HJ, Guan Y, Shi M, Yang J, Li DP, Zhang Y (2014) Chronic intermittent hypobaric hypoxia ameliorates ischemia/reperfusion-induced calcium overload in heart via Na/Ca2+ exchanger in developing rats. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 34:313–324
Mishra P, Senthivinayagam S, Rangasamy V, Sondarva G, Rana B (2010) Mixed lineage kinase-3/JNK1 axis promotes migration of human gastric cancer cells following gastrin stimulation. Mol Endocrinol 24:598–607
Ola A, Kerkela R, Tokola H, Pikkarainen S, Skoumal R, Vuolteenaho O, Ruskoaho H (2010) The mixed-lineage kinase 1-3 signalling pathway regulates stress response in cardiac myocytes via GATA-4 and AP-1 transcription factors. Br J Pharmacol 159:717–725
Qin C, Zhou S, Xiao Y, Chen L (2014) Erythropoietin enhances mitochondrial biogenesis in cardiomyocytes exposed to chronic hypoxia through Akt/eNOS signalling pathway. Cell Biol Int 38:335–342
Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870–874
Zhou F, Xu Y, Hou XY (2014) MLK3-MKK3/6-P38MAPK cascades following N-methyl-D-aspartate receptor activation contributes to amyloid-beta peptide-induced apoptosis in SH-SY5Y cells. Journal of neuroscience research.
Acknowledgements
The present work was supported by the project of youth scientific and technological innovation in Chengdu Military General Hospital (No. 41732C11K) and technological project of Sichuan health and family planning commission (No. 16PJ022).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures were approved by hospital’s Human Ethical Committee and were performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s13105-017-0593-x.
Electronic supplementary material

Supplementary Fig 1
Transfection agents information and efficiency detection. (A) The transfection carrier atlas. (B) The constructed shRNA was encoded with green fluorescence protein (GFP). Almost all transfected cardiomyocytes were emitting green light by using a fluorescence microscope. Expression of MLK3 mRNA in MLK3-shRNA group was only 11% of that in scramble-shRNA group (C) (#p < 0.05, n = 9), while protein expression was decreased to 36% once MLK3 shRNA was transfected (D) (#p < 0.05, n = 3). Values were means ± SEM. (JPEG 615 kb)
Rights and permissions
About this article
Cite this article
He, S., Liu, S., Wu, X. et al. Protective role of downregulated MLK3 in myocardial adaptation to chronic hypoxia. J Physiol Biochem 73, 371–380 (2016). https://doi.org/10.1007/s13105-017-0561-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-017-0561-5