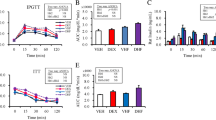
Abstract
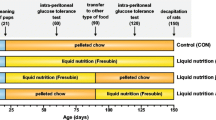
The aim of the study was to analyze the phenotypic and epigenetic changes induced by the shift to a chow diet after an obesogenic environment. Animals were randomized to fed chow (control group) or high-fat–sucrose diet (HFS). After 10 weeks, half of the rats fed with HFS diet were reassigned to a chow diet (rest group) while the other half continued with the obesogenic diet (HFS group) until week 20. Changes in fat content, biochemical profile, and DNA methylation levels of several gene promoters from retroperitoneal adipocytes were analyzed. HFS diet intake for 10 weeks induced obese phenotype in the animals, increasing body weight and fat content. These effects were maintained until the end of the trial in HFS group, where an increase in liver fat content, a modification of lipid profile, and retroperitoneal adipose tissue hypertrophy were also observed. Changing the dietary pattern reversed these parameters. Epigenetic analysis showed that HFS diet intake for 20 weeks hypermethylated several CpG sites (6.7 and 29.30) and hypomethylated CpG site 15 from leptin gene promoter. Moreover, the obesogenic diet also hypomethylated CpG site 1 from Fasn (fatty acid synthase) gene promoter, without changes on Ppargc1a (peroxisome proliferator-activated receptor gamma coactivator 1-alpha), Srebf1 (sterol regulatory element-binding transcription factor 1), and aquaporin 7. Shifting to a chow diet reverted HFS-induced DNA methylation levels of some CpG sites of leptin promoter. Changing the dietary pattern hypomethylated a CpG site of Srebf1 and hypermethylated other CpGs on Ppargc1a and Fasn promoter. This study shed light on the reversibility of phenotypical and epigenetic changes induced by a HFS diet intake.



Similar content being viewed by others
References
Alibegovic AC, Sonne MP, Hojbjerre L, Bork-Jensen J, Jacobsen S, Nilsson E, Faerch K, Hiscock N, Mortensen B, Friedrichsen M, Stallknecht B, Dela F, Vaag A (2010) Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol Endocrinol Metab 299:E752–763
Allison DB, Pi-Sunyer FX (1995) Obesity treatment: examining the premises. Endocr Pract 1:353–364
Amengual-Cladera E, Llado I, Gianotti M, Proenza AM (2012) Sex differences in the effect of high-fat diet feeding on rat white adipose tissue mitochondrial function and insulin sensitivity. Metabolism 61:1108–1117
Bird A (2007) Perceptions of epigenetics. Nature 447:396–398
Bock C, Beerman I, Lien WH, Smith ZD, Gu H, Boyle P, Gnirke A, Fuchs E, Rossi DJ, Meissner A (2012) DNA methylation dynamics during in vivo differentiation of blood and skin stem cells. Mol Cell 47:633–647
Bondia-Pons I, Boque N, Paternain L, Santamaria E, Fernandez J, Campion J, Milagro F, Corrales F, Martinez JA (2011) Liver proteome changes induced by a short-term high-fat sucrose diet in wistar rats. J Nutrigenet Nutrigenomics 4:344–353
Boque N, Campion J, Paternain L, Garcia-Diaz DF, Galarraga M, Portillo MP, Milagro FI, Ortiz de Solorzano C, Martinez JA (2009) Influence of dietary macronutrient composition on adiposity and cellularity of different fat depots in Wistar rats. J Physiol Biochem 65:387–395
Bouchard L, Rabasa-Lhoret R, Faraj M, Lavoie ME, Mill J, Perusse L, Vohl MC (2010) Differential epigenomic and transcriptomic responses in subcutaneous adipose tissue between low and high responders to caloric restriction. Am J Clin Nutr 91:309–320
Bouchard L, Thibault S, Guay SP, Santure M, Monpetit A, St-Pierre J, Perron P, Brisson D (2010) Leptin gene epigenetic adaptation to impaired glucose metabolism during pregnancy. Diabetes Care 33:2436–2441
Brons C, Jacobsen S, Nilsson E, Ronn T, Jensen CB, Storgaard H, Poulsen P, Groop L, Ling C, Astrup A, Vaag A (2010) Deoxyribonucleic acid methylation and gene expression of PPARGC1A in human muscle is influenced by high-fat overfeeding in a birth-weight-dependent manner. J Clin Endocrinol Metab 95:3048–3056
Brug J (2007) The European charter for counteracting obesity: a late but important step towards action. Observations on the WHO-Europe ministerial conference, Istanbul, November 15–17, 2006. Int J Behav Nutr Phys Act 4:11
Campion J, Milagro FI, Martinez JA (2009) Individuality and epigenetics in obesity. Obes Rev 10:383–392
Chen J, Yang XJ, Xia D, Wegner J, Jiang Z, Zhao RQ (2008) Sterol regulatory element binding transcription factor 1 expression and genetic polymorphism significantly affect intramuscular fat deposition in the longissimus muscle of Erhualian and Sutai pigs. J Anim Sci 86:57–63
Clifton P (2012) Effects of a high protein diet on body weight and comorbidities associated with obesity. Br J Nutr 108(Suppl 2):S122–129
Cordero P, Campion J, Milagro FI, Goyenechea E, Steemburgo T, Javierre BM, Martinez JA (2011) Leptin and TNF-alpha promoter methylation levels measured by MSP could predict the response to a low-calorie diet. J Physiol Biochem 67:463–470
Cordero P, Gomez-Uriz AM, Campion J, Milagro FI, Martinez JA (2012) Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr 8:105–113
Fontaine KR (2001) Weight loss and health-related quality of life. Am J Manag Care 7:926–927
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Galarraga M, Campión J, Muñoz-Barrutia A, Boqué N, Moreno H, Martínez JA, Milagro F, Ortiz-de-Solórzano C. (2012) Adiposoft: an automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res 53:2791–2796
Gervin K, Vigeland MD, Mattingsdal M, Hammero M, Nygard H, Olsen AO, Brandt I, Harris JR, Undlien DE, Lyle R (2012) DNA methylation and gene expression changes in monozygotic twins discordant for psoriasis: identification of epigenetically dysregulated genes. PLoS Genet 8:e1002454
Gordon L, Joo JE, Powell JE, Ollikainen M, Novakovic B, Li X, Andronikos R, Cruickshank MN, Conneely KN, Smith AK, Alisch RS, Morley R, Visscher PM, Craig JM, Saffery R (2012) Neonatal DNA methylation profile in human twins is specified by a complex interplay between intrauterine environmental and genetic factors, subject to tissue-specific influence. Genome Res 22:1395–1406
Hambly C, Speakman JR (2005) Contribution of different mechanisms to compensation for energy restriction in the mouse. Obes Res 13:1548–1557
Jacobsen SC, Brons C, Bork-Jensen J, Ribel-Madsen R, Yang B, Lara E, Hall E, Calvanese V, Nilsson E, Jorgensen SW, Mandrup S, Ling C, Fernandez AF, Fraga MF, Poulsen P, Vaag A (2012) Effects of short-term high-fat overfeeding on genome-wide DNA methylation in the skeletal muscle of healthy young men. Diabetologia 55:3341–3349
Jiang M, Zhang Y, Liu M, Lan MS, Fei J, Fan W, Gao X, Lu D (2011) Hypermethylation of hepatic glucokinase and L-type pyruvate kinase promoters in high-fat diet-induced obese rats. Endocrinology 152:1284–1289
Jousse C, Parry L, Lambert-Langlais S, Maurin AC, Averous J, Bruhat A, Carraro V, Tost J, Letteron P, Chen P, Jockers R, Launay JM, Mallet J, Fafournoux P (2011) Perinatal undernutrition affects the methylation and expression of the leptin gene in adults: implication for the understanding of metabolic syndrome. FASEB J 25:3271–3278
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Kochan Z (2003) Increased lipogenic potential of rat adipose tissue after repeated dieting—the role of SREBP-1 transcription factor. Cell Mol Biol Lett 8:901–909
Lemonnier D (1972) Effect of age, sex, and sites on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J Clin Invest 51:2907–2915
Ling C, Del Guerra S, Lupi R, Ronn T, Granhall C, Luthman H, Masiello P, Marchetti P, Groop L, Del Prato S (2008) Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 51:615–622
Logue J, Thompson L, Romanes F, Wilson DC, Thompson J, Sattar N (2010) Management of obesity: summary of SIGN guideline. BMJ 340:c154
Lomba A, Martinez JA, Garcia-Diaz DF, Paternain L, Marti A, Campion J, Milagro FI (2010) Weight gain induced by an isocaloric pair-fed high fat diet: a nutriepigenetic study on FASN and NDUFB6 gene promoters. Mol Genet Metab 101:273–278
Lomba A, Milagro FI, Garcia-Diaz DF, Marti A, Campion J, Martinez JA (2010) Obesity induced by a pair-fed high fat sucrose diet: methylation and expression pattern of genes related to energy homeostasis. Lipids Health Dis 9:60
Maeda N (2012) Implications of aquaglyceroporins 7 and 9 in glycerol metabolism and metabolic syndrome. Mol Aspects Med 33:665–675
Martinez JA (2000) Body-weight regulation: causes of obesity. Proc Nutr Soc 59:337–345
Martinez JA, Cordero P, Campion J, Milagro FI (2012) Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc Nutr Soc 71:276–283
Milagro FI, Campion J, Cordero P, Goyenechea E, Gomez-Uriz AM, Abete I, Zulet MA, Martinez JA (2011) A dual epigenomic approach for the search of obesity biomarkers: DNA methylation in relation to diet-induced weight loss. FASEB J 25:1378–1389
Milagro FI, Campion J, Garcia-Diaz DF, Goyenechea E, Paternain L, Martinez JA (2009) High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem 65:1–9
Milagro FI, Mansego ML, De Miguel C, Martínez JA (2012) Dietary factors, epigenetic modifications and obesity outcomes: Progresses and perspectives. Mol Aspects Med. doi:10.1016/j.mam.2012.06.010
Mirhashemi F, Scherneck S, Kluth O, Kaiser D, Vogel H, Kluge R, Schurmann A, Neschen S, Joost HG (2011) Diet dependence of diabetes in the New Zealand Obese (NZO) mouse: total fat, but not fat quality or sucrose accelerates and aggravates diabetes. Exp Clin Endocrinol Diabetes 119:167–171
Misra A, Khurana L (2008) Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 93:S9–30
Morgan HD, Santos F, Green K, Dean W, Reik W (2005) Epigenetic reprogramming in mammals. Hum Mol Genet 14 Spec No 1:R47–58
Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM (2010) Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring) 18:1652–1659
Orzano AJ, Scott JG (2004) Diagnosis and treatment of obesity in adults: an applied evidence-based review. J Am Board Fam Pract 17:359–369
Park LK, Friso S, Choi SW (2012) Nutritional influences on epigenetics and age-related disease. Proc Nutr Soc 71:75–83
Paternain L, Batlle MA, De la Garza AL, Milagro FI, Martínez JA, Campión J (2012) Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat–sucrose diet in prenatally stressed female rats. Neuroendocrinology. doi:10.1159/000341684
Paternain L, de la Garza AL, Batlle MA, Milagro FI, Martínez JA, Campión J (2012) Prenatal stress increases the obesogenic effects of a high-fat–sucrose diet in adult rats in a sex-specific manner. Stress. doi:10.3109/10253890.2012.707708
Pinnick KE, Karpe F (2011) DNA methylation of genes in adipose tissue. Proc Nutr Soc 70:57–63
Sato M, Kawakami T, Kondoh M, Takiguchi M, Kadota Y, Himeno S, Suzuki S (2010) Development of high-fat-diet-induced obesity in female metallothionein-null mice. FASEB J 24:2375–2384
Sookoian S, Rosselli MS, Gemma C, Burgueno AL, Fernandez Gianotti T, Castano GO, Pirola CJ (2010) Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor gamma coactivator 1alpha promoter. Hepatology 52:1992–2000
Sun L, Yang Z, Jin F, Zhu XQ, Qu YC, Shi XH, Wang L (2006) The Gly482Ser variant of the PPARGC1 gene is associated with type 2 diabetes mellitus in northern Chinese, especially men. Diabet Med 23:1085–1092
Volkmar M, Dedeurwaerder S, Cunha DA, Ndlovu MN, Defrance M, Deplus R, Calonne E, Volkmar U, Igoillo-Esteve M, Naamane N, Del Guerra S, Masini M, Bugliani M, Marchetti P, Cnop M, Eizirik DL, Fuks F (2012) DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J 31:1405–1426
Weyer C, Walford RL, Harper IT, Milner M, MacCallum T, Tataranni PA, Ravussin E (2000) Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. Am J Clin Nutr 72:946–953
Widiker S, Karst S, Wagener A, Brockmann GA (2010) High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet 51:193–197
Zhang Y, Chen FQ, Sun YH, Zhou SY, Li TY, Chen R (2011) Effects of DNMT1 silencing on malignant phenotype and methylated gene expression in cervical cancer cells. J Exp Clin Cancer Res 30:98
Acknowledgments
The authors thank the “Línea Especial” (LE/97, University of Navarra, Spain) for financial support. The technical assistance of Almudena Aguado and Alexandra Claire Simpson is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1
a Nucleotide sequence of the CpG island in the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Ppargc1a) promoter region showing individual CpG dinucleotides. b The effect of HFS diet intake and shifting to a chow diet in the methylation levels of individual CpG dinucleotides in the Ppargc1a promoter. Data are mean ± SEM. Statistical analyses were performed using one-way ANOVA test and DMS post hoc test. Different letters (a, b, c) indicate significant differences between groups of at least p < 0.05. Ppargc1a, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; HFS, high-fat–sucrose; rest, animals shifted from HFS to chow diet; Chr., chromosome; NA, not available (PDF 114 kb)
Figure 2
a Nucleotide sequence of the CpG island in the leptin promoter region showing individual CpG dinucleotides. b The effect of HFS diet intake and shifting to a chow diet in the methylation levels of individual CpG dinucleotides in the leptin promoter. Data are mean ± SEM. Statistical analyses were performed using one-way ANOVA test and DMS post hoc test. Different letters (a, b) indicate significant differences between groups of at least p < 0.05. t indicates a trend to significance. HFS, high-fat–sucrose; rest, animals shifted from HFS to chow diet; Chr., chromosome; NA, not available (PDF 147 kb)
Figure 3
a Nucleotide sequence of the CpG island in the sterol regulatory element-binding transcription factor 1 (Srebf1) promoter region showing individual CpG dinucleotides. b The effect of HFS diet intake and shifting to a chow diet in the methylation levels of individual CpG dinucleotides in the Srebf1 promoter. Data are mean ± SEM. Statistical analyses were performed using one-way ANOVA test and DMS post hoc test. Different letters (a, b) indicate significant differences between groups of at least p < 0.05. t indicates a trend to significance. HFS, high-fat–sucrose; rest, animals shifted from HFS to chow diet; Chr., chromosome; NA, not available (PDF 131 kb)
Figure 4
a Nucleotide sequence of the CpG island in the Fatty acid synthase (Fasn) promoter region showing individual CpG dinucleotides. b The effect of HFS diet intake and shifting to a chow diet in the methylation levels of individual CpG dinucleotides in the Fasn promoter. Data are mean ± SEM. Statistical analyses were performed using One-way ANOVA test and DMS post hoc test. Different letters (a, b) indicate significant differences between groups of at least p < 0.05. HFS, high-fat–sucrose; rest, animals shifted from HFS to chow diet; Chr., chromosome; NA, not available (PDF 135 kb)
Figure 5
a Nucleotide sequence of the CpG island in the Aquaporin 7 (Aqp 7) promoter region showing individual CpG dinucleotides. b The effect of HFS diet intake and shifting to a chow diet in the methylation levels of individual CpG dinucleotides in the Aqp 7 promoter. Data are mean ± SEM. Statistical analyses were performed using one-way ANOVA test and no statistically significant changes were observed. HFS, high-fat–sucrose; rest, animals shifted from HFS to chow diet; Chr., chromosome; NA, not available (PDF 111 kb)
Rights and permissions
About this article
Cite this article
Uriarte, G., Paternain, L., Milagro, F.I. et al. Shifting to a control diet after a high-fat, high-sucrose diet intake induces epigenetic changes in retroperitoneal adipocytes of Wistar rats. J Physiol Biochem 69, 601–611 (2013). https://doi.org/10.1007/s13105-012-0231-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-012-0231-6