Abstract
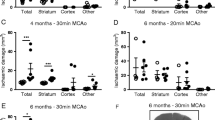
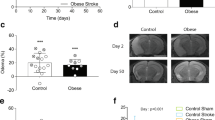
Obesity is associated with chronic peripheral inflammation, is a risk factor for stroke, and causes increased infarct sizes. To characterize how obesity increases infarct size, we fed a high-fat diet to wild-type C57BL/6J mice for either 6 weeks or 15 weeks and then induced distal middle cerebral artery strokes. We found that infarct expansion happened late after stroke. There were no differences in cortical neuroinflammation (astrogliosis, microgliosis, or pro-inflammatory cytokines) either prior to or 10 h after stroke, and also no differences in stroke size at 10 h. However, by 3 days after stroke, animals fed a high-fat diet had a dramatic increase in microgliosis and astrogliosis that was associated with larger strokes and worsened functional recovery. RNA sequencing revealed a dramatic increase in inflammatory genes in the high-fat diet-fed animals 3 days after stroke that were not present prior to stroke. Genetic pathways unique to diet-induced obesity were primarily related to adaptive immunity, extracellular matrix components, cell migration, and vasculogenesis. The late appearance of neuroinflammation and infarct expansion indicates that there may be a therapeutic window between 10 and 36 h after stroke where inflammation and obesity-specific transcriptional programs could be targeted to improve outcomes in people with obesity and stroke.







Similar content being viewed by others
References
Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808.
Heiss WD. The ischemic penumbra: how does tissue injury evolve? Ann N Y Acad Sci. 2012;1268:26–34.
Cekanaviciute E, Fathali N, Doyle KD, Williams AM, Han J, Buckwalter MS. Astrocytic transformating growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia. 2014;62(8):1227–40.
Xu H. Obesity and metabolic inflammation. Drug Discov Today. 2013;10(1–2):21–5.
N. I. o. H. U.S. Department of Health and Human services, National Institute of Diabetes and Digestive and Kidney Diseases, Overweight & obesity statistics, Retrieved from https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity, 2010.
Strazullo P, D'Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41(5):418–26.
Li P, Lu M, Nguyen MTA, Bae EJ, Chapman J, Feng D, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285(20):15333–45.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84.
Lumeng CN, DelProposto J, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–46.
Weissberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante JW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808.
Galvez I, Martin-Cordero L, Hinchado MD, Alvarez-Barrientos A, Ortega E. Anti-inflammatory effect of β2 adrenergic stimulation on circulating monocytes with a pro-inflammatory state in high-fat diet-induced obesity. Brain Behav Immun. 2019;19:30169–72.
Wisse B. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. JASN. 2004;15(11):2792–800.
Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):4615–55.
Coelho M, Oliveira T, Fernanes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9(2):191–200.
Pepping JK, Freeman LR, Gupta S, Keller JN, Bruce-Keller AJ. NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am J Physiol Endocrinol Metab. 2013;304:E392–404.
Takechi R, Pallebage-Gamarallage MM, Giles C, Mamo JC. Nutraceutical agents with anti-inflammatory properties prevent dietary saturated-fat induced disturbances in blood-brain barrier function in wild-type mice. J Neuroinflammation. 2013;19(10):73–85.
Costa de Aquino C, et al. Effect of hypoproteic and high-fat diets on hippocampal blood-brain barrier permeability and oxidative stress. Front Nutr. 2018;5:131–41.
Valdearcose M, et al. Microglial inflammatory signal orchestrates he hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017;26:185–97.
Buckman LB, Thompson MM, Moreno HN, Ellacot KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol. 2013;521:322–33.
Noronha SSR, et al. Association of high-fat diet with neuroinflammation, anxiety-like defensive behavioral responses, and altered thermoregulatory responses in male rats. Brain Behav Immun. 2019;19:30137–10349.
Rahman MH, Kim MS, Lee IK, Yu R, Suk K. Interglial crosstalk in obesity-induced hypothalamic inflammation. Front Neurosci. 2018;13(12):939–48.
Lainez NM, et al. Diet-induced obesity elicits macrophage infiltration and reduction in spine density in the hypothalami of male but not female mice. Front Immunol. 2018;11(9):1992–2008.
Lee CH, Kim HJ, Lee YS, Kang GM, Lim HS, Lee SH, et al. Hypothalamic macrophage inducible nitric oxide synthase mediates obesity-associated hypothalamic inflammation. Cell Rep. 2018;25(4):934–46.
Wu M, et al. Obesity exacerbates rat cerebral ischemic injury through enhancing ischemic adiponectin-containing neuronal apoptosis. Mol Neurobiol. 2016;53:3702–13.
Yang Z, Chen Y, Zhang Y, Yiang Y, Fang X, Xu J. Sevoflurane postconditioning against cerebral ischemic neuronal injury is abolished in diet-induced obesity: role of brain mitochondrial KATP channels. Mol Med Rep. 2014;8:843–50.
Deutsch C, Portik-Dobos D, Smith AD, Ergul A, Dorrance AM. Diet-induced obesity causes cerebral vessel remodeling and increase the damage cause by ischemic stroke. Microvasc Res. 2009;78:100–6.
Yan BC, Park JH, Ahn JH, Kim IH, Lee JC, Yoo KY, et al. Effects of high-fat diet on neuronal damage, gliosis, inflammatory process, and oxidative stress in the hippocampus induced by transient cerebral ischemia. Neurochem Res. 2014;39(12):2465–78.
Cao X, Du Y, Yan J, Hu X. Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation, and neuronal apoptosis in MCAO/reperfusion rats. Exp Brain Res. 2015;233:2753–65.
Kim E, Yang J, Park KW, Cho S. Inhibition of VEGF signaling reduces diabetes-exacerbated brain swelling, but not infarct size, in large cerebral infarction in mice. Transl Stroke Res. 2018;9(5):540–8.
Haley MJ, Krishnan S, Burrows D, de Hoog L, Thakrar J, Schiessl I, et al. Acute high-fat feeding leads to disruptions in glucose homeostasis and worsens stroke outcome. J Cereb Blood Flow Metab. 2019;39(6):1026–37.
Kim E, Yang J, Woo Park K, Cho S. Preventative, but not post-stroke, inhibition of CD36 attenuates brain swelling in hyperlipidemic stroke. J Cereb Blood Flow Metab. 2019;15:1–8.
McColl BW, Rose N, Robson FH, Rothwell NJ, Lawrence CB. Increased brain microvascular MMP-9 and incidence of haemorrhagic transformation in obese mice after experimental stroke. J Cereb Blood Flow Metab. 2010;30(2):267–72.
Maysami S, Haley MJ, Gorenkova N, Krishnan B, McColl BW, Lawrence CB. Prolonged diet-induced obesity in mice modifies the inflammatory response and leads to worsened outcomes after stroke. J Neuroinflammation. 2015;12:140–52.
King VL, Hatch NW, Chan HW. A murine model of obesity with accelerated athersclerosis. Obesity. 2009;18(1):35–41.
Y. Hang et al., The MafA transcription factor becomes essential to islet β-cells soon after birth, Diabetes, p. DB_131001, 2014.
Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischemia in the rat: description of technique and early pathological consequences following middle cerebal artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60.
Doyle KP, Fathali N, Siddiqui AM, Buckwalter BL. Distal hypoxic stroke: a new mouse model of stroke with high throughput, low variability, and a quantifiable functional deficit. J Neurosci Methods. 2012;207:31–40.
Nouraee C, Fisher M, di Napoli M, Salazar P, Farr TD, Jafarli A, et al. A brief review of edema-adjusted infarct volume measurement techniques for rodent focal cerebral ischemia models with practical recommendations. Journal of Vascular and Interventional Neurology. 2019;10:38–45.
Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2:13–24.
Shah AM, et al. Optogenetic neuronal stimulation of the lateral cerebellar nucleus promotes persistent functional recovery after stroke. Sci Rep. 2017;1(7):1–11.
Cheng MY, Aswendt M, Steinberg GK. Optogentic approaches to target specific neural circuits in post-stroke recovery. Neurotherapeutics. 2016;13(2):325–40.
Cheng MY, et al. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci U S A. 2014;2(111):12913–8.
Wang J, Lin X, Mu Z, Shen F, Zhang L, Xie Q, et al. Rapamycin increases collateral circulation in rodent brain after focal ischemia as detected by multiple modality dynamic imaging. Theranostics. 2019;9(17):4923–34.
M. D. Robinson, D. J. McCarthy, and G. K. Smyth, edgeR: a bioconductor package for differential expression analysis of digital gene expression data, Bioinformatics, vol. 26, no. 1, pp. 139–140, 2010.
McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40(10):4288–97. https://doi.org/10.1093/nar/gks042.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504.
Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:W214–20.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550–71.
R. C. Team, R: a language and environment for statistical computing. R Foundational Computing, Vienna, Austria, 2013. [Online]. Available: http://www.R-project.org/.
Bates D, Machler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48.
Young ME, Clark MH, Goffus A, Hoane MR. Mixed effects modeling of Morris water maze data: advantages and cautionary notes. Learn Motiv. 2008;40:160–77.
Zeng H, Vaka VR, He X, Booz GW, Chen J. High-fat diet induces cardiac remodeling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med. 2015;19(8):1847–56.
Ono-Moore KD, Ferguson M, Blackburn ML, Issafras H, Adams SH. Application of an in vivo hepatic triacylglycerol production method in the setting of a high-fat diet in mice. Nutrients. 2016;9:1–16.
M. Cao et al., Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice, Stem Cell Research & Therapy, vol. 6, no. 1, p. 208, 2015.
L. M. Williams et al., The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases, PLoS One, vol. 9, no. 8, p. e106159, 2014.
K. L. Lechtenberg, S. T. Meyer, J. B. Doyle, T. C. Peterson, and M. S. Buckwalter, Augmented beta2-adrenergic signaling dampens the neuroinflamatory response following ischemic stroke and increases stroke size, Journal of Neuroinflammation, vol. 16, no. 112–130, 2019.
Deng J, Zhang J, Feng C, Xiong L, Zuo Z. Critical role of matrix metalloprotease-9 in chronic high-fat diet induced cerebral vascular remodelling and increase ischaemic brain injury in mice. Cardiovasc Res. 2014;103:473–84.
Li W, Prakash R, Chawla D, du W, Didion SP, Filosa JA, et al. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Integr Comp Physiol. 2013;304:R1001–8.
Dhungana H, et al. Western-type diet modulates inflammatory response and impairs functional outcome following permanent middle cerebral artery occlusion in aged mice expressing the human apolipoprotein E4 allele. J Neuroinflammation. 2013;10:102–15.
Kim E, Tolhurst AT, Qin LY, Chen X, Febbraio M, Cho S. CD36/fatty acid translocase, an inflammatory mediator, in involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci. 2008;28(18):4661–7670.
Kim E, Yang J, Woo Park K, Cho S. Preventative, but not post-stroke, inhibition of CD36 attenuates brain swelling in hyperlipidemic stroke. J Cereb Blood Flow Metab. 2019;15:1–10.
Haley MJ, Lawrence CB. Obesity and stroke: can we translate from rodents to patients? J Cereb Blood Flow Metab. 2016;36(12):2007–21.
Kim E, Tolhurst AT, Cho S. Deregulation of inflammatory response in the diabetic condition is associated with increased ischemic brain injury. J Neuroinflammation. 2014;1(11):1–9.
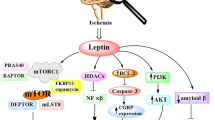
Saiinz N, Barrenetxe J, Moreno-Aliagra MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64(1):35–46.
Lubjuhn J, Gastens A, von Wilpert G, Bargiotas P, Herrmann O, Murikinati S, et al. Functional testing in a mouse stroke model induced by occlusion of the distal middle cerebral artery. J Neurosci Methods. 2009;184(1):95–103.
F. Luo et al., Cuprizone-induced demyelination under physiological and post-stroke condition leads to decreased neurogenesis response in adult mouse brain. Exp Neurol, vol. 326, 2020.
Calvalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57(14):1511–22.
Stenmark KR, Yeager ME, el Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, et al. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol. 2013;75:23–47.
Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14(1):5–12.
Kim E, Cho S. Microglia and monocyte-derived macrophages in stroke. Neurotherapeutics. 2016;13(4):702–18.
C. J. Smith, C. B. Lawrence, B. Rodrguez-Grande, K. J. Kovacs, J. M. Pradillo, and A. Denes, The immune system in stroke: clinical challenges and their translation to experimental research, J Neuroimmune Pharmacol, vol. 8, no. 867–887, 4, 2013.
Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32.
Guillemot-Legris O, Masquelier J, Everard A, Cani PD, Alhouayek M, Muccioli GG. High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J Neuroinflammation. 2016;13(1):206–17.
Wang S, Huang XF, Zhang P, Wang H, Zhang Q, Yu S, et al. Chronic rhein treatment improves recognition memory in high-fat diet-induced obese male mice. J Nutr Biochem. 2016;36:42–50.
Tarantini S, et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood–brain barrier disruption, neuroinflammation, amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2017;73(7):853–63.
Jayaraman A, Lent-Schochet D, Pike CJ. Diet-induced obesity and low testosterone increase neuroinflammation and impair neural function. J Neuroinflammation. 2014;11:162–76.
J. L. Carlin, N. Grissom, Z. Ying, F. Gomez-Pinilla, and T. M. Reyes, Voluntary exercise blocks Western diet-induced gene expression of the chemokines CXCL10 and CCL2 in the prefrontal cortex. Brain Behav Immun, vol. 58, no. 82–90, 2016.
Rodriguez EM, Blazquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private millieus: the former opens to the portal blood and the latter to the cerebral spinal fluid. Peptides. 2010;31(4):757–76.
S. Kalin, F. L. Heppner, I. Bechmann, M. Prinz, M. H. Tschop, and C. Yi, Hypothalamic innate immune reaction in obesity, Nature Reviews Endocrinology, vol. 11, no. 339–351, 2015.
Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKB/NfkB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73.
Waise TM, et al. One-day high-fat diet induced inflammation in the nodose ganglion and the hypothalamus. Biochem Biophys Res Commun. 2015;464(4):1157–62.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–62.
Haley MJ, Mullard G, Hollywood KA, Cooper GJ, Dunn WB, Lawrence CB. Adipose tissue and metabolic and inflammatory responses to stroke are altered in obese mice. Dis Model Mech. 2017;10(10):1229–43.
Morris DL, Cho KW, DelProposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, et al. Adipose tissue macrophages function as antigen-presenting cells and regulated adipose tissue CD4+ cells in mice. Diabetes. 2013;62:2762–72.
Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propogation, and remodeling. Future Lipidol. 2008;3(5):545–56.
Zheng C, et al. CD11b regulates obesity-induced insulin resistence via limiting alternative activation and proliferation of adipose tissue macrophages. Proc Natl Acad Sci U S A. 2015;111(52):7239–48.
Cho KW, et al. An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity induced inflammation. Cell Rep. 2009;9:605–17.
D. Dicker, M. A. Salook, D. Marcoviciu, M. Djaldetti, and H. Bessler, Role of peripheral blood mononuclear cells in the predisposition of obese individuals to inflammation and infection, Obesity Facts, vol. 6, no. 146–151, 2013.
Catalan V, et al. Peripheral mononuclear blood cells contribute to the obesity-associated inflammatory state independently of glycemic status: involvement of the novel proinflammatory adipokines chemerin, chitinase-3-like protein 1, lipocalin-2 and osteopontin. Genes and Nutrition. 2015;10(11):1–11.
Tsai AS, Berry K, Beneyto MM, Gaudilliere D, Ganio EA, Culos A, et al. A year-long immune profile of the systemic response in acute stroke survivors. Brain. 2019;142(4):978–91.
Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab. 2015;26(7):357–66.
Seo BR, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;19(7):1–11.
Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15(3):139–54.
Dzyubenko E, Manrique-Castano D, Kleinschnitz C, Faissner A, Hermann DM. Role of immune responses for extracellular matrix remodeling in the ischemic brain. Ther Adv Neurol Disord. 2018;11:1–11.
Oliveira Dias D, Gortiz C. Fibrotic scarring following lesions to the central nervous system. Matrix Biol. 2018;68-69:561–70.
Deng T, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;5(17):411–22.
Doyle KP, Buckwalter BL. Does B lymphocyte-mediated autoimmunity contribute to post-stroke dementia? Brain Behav Immun. 2017;64:1–8.
Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117(9):2362–8.
Wagner IJ, et al. Obesity impairs wound closure through a vasculogenic mechanism. Wound Repair Regen. 2012;20(4):512–22.
M. J. Haley et al., Stroke induces prolonged changes in lipid metabolism, the liver, and body composition in mice, Translational Stroke Research, pp. 1–14, 2019, doi: https://doi.org/10.1007/s12975-019-00763-2.
Liz MA, Mar FM, Franquinho F, Sousa M. Aboard transthyretin: from transport to cleavage. Life. 2010;62(6):429–35.
Lin HY, et al. Molecular basis for certain neuroprotective effects of thyroid hormone. Front Mol Neurosci. 2011;14(4):1–6.
Santos SD, Lambertsen KL, Clausen BH, Akinc A, Alvarez R, Finsen B, et al. CSF transthyretin neuroprotection in a mouse model of brain ischemia. J Neurochem. 2010;115:1434–44.
Puig-Kroger A, Sierra-Filardi E, Dominguez-Soto A, Samaniego R, Corcuera MT, Gomez-Aguado F, et al. Folate receptor β is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69(24):9395–403.
L. Oesch, T. Tatlisumak, M. Arnold, and H. Sarikaya, Obesity paradox in stroke - myth or reality? A systematic review. PLoS One, vol. 12, no. 3, p. e0171334, 2017.
Mosser RE, Maulis MF, Moullé VS, Dunn JC, Carboneau BA, Arasi K, et al. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Endocrinol Metab. 2015;308(7):E573–82.
Heydemann A. An overview of murine high fat diet as a model for type 2 diabetes mellitus. Journal of Diabetes Research. 2016;2016:1–14.
Acknowledgments
This work used the Genome Sequencing Service Center by Stanford Center for Genomics and Personalized Medicine Sequencing Center, supported by NIH S10OD020141.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke, RO1 NS067132 (Buckwalter) and NRSA F32 NS089162 (Peterson), and by a Brain Health Frontiers Award from the American Heart Association and the Paul Allen Frontiers Group. (Buckwalter)
Author information
Authors and Affiliations
Contributions
Marion S. Buckwalter designed and performed the research, analyzed the data, and wrote the paper. Todd C. Peterson designed and performed the research, analyzed the data, and wrote the paper. Kendra J. Lechtenberg designed and performed the research and analyzed the data. Brian D. Piening designed and performed the research and analyzed the data. Tawaun A. Lucas designed and performed the research and analyzed the data. Eric Wei analyzed the data. Hassan Chaib analyzed the data. Alexa K. Dowdell analyzed the data. Michael Snyder designed the research and analyzed the data.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use for animals were followed. All use and care of animals was approved by UNCW IACUC protocol number A1819-01.
Informed Consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary material for this paper can be found at the journal website.
ESM 1
(PDF 633 kb)
Rights and permissions
About this article
Cite this article
Peterson, T.C., Lechtenberg, K.J., Piening, B.D. et al. Obesity Drives Delayed Infarct Expansion, Inflammation, and Distinct Gene Networks in a Mouse Stroke Model. Transl. Stroke Res. 12, 331–346 (2021). https://doi.org/10.1007/s12975-020-00826-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-020-00826-9