Abstract
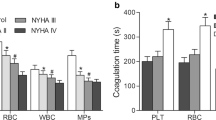
The role of phosphatidylserine (PS)-mediated procoagulant activity (PCA) in stroke remains unclear. To ascertain this role, early dynamic evolution of PS exposure on blood cells and released microparticles (MPs) and the corresponding PCA were evaluated in patients with acute ischemic stroke (AIS). Flow cytometry analyses revealed that initial levels of PS exposure on erythrocyte, platelet, and leukocyte were 2.40-, 1.36-, and 1.38-fold higher, respectively, in AIS than the risk factor-matched (RF) controls. Concomitantly, total PS+ MPs were increased in AIS (1949 ± 483/μl) compared with the RF group (1674 ± 387/μl; P = 0.019) and healthy controls (1052 ± 179/μl; P < 0.001). Specifically, PS+ erythrocytes gradually increased within 1 week. PS+ platelets and MPs peaked at 24 h and declined at 7 days, while PS+ leukocytes were markedly elevated at 24 h. Further, PS exposure on blood cells and MPs in stroke resulted in shortened clotting time with an accompanying increase in FXa and thrombin formation significantly. Treatment with lactadherin, a PS antagonist, delayed the coagulation time by approximately 20 % and blocked the generation of FXa and thrombin by about 50 %. Furthermore, initial counts of PS+ platelets and platelet MPs significantly correlated with stroke severity. Thrombin generation promoted by platelets and MPs at 12 h was significantly higher in patients with cardioembolism than in patients without. The thrombophilic susceptibility of AIS patients can be partly ascribed to PS exposure on blood cells and the release of MPs. Our studies identify PS exposure as a potentially novel therapeutic target in the treatment of AIS.




Similar content being viewed by others
References
Berge E, Friis P, Sandset PM. Hemostatic activation in acute ischemic stroke. Thromb Res. 2001;101:13–21.
Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood. 2008;112:3555–62.
Carcaillon L, Alhenc-Gelas M, Bejot Y, et al. Increased thrombin generation is associated with acute ischemic stroke but not with coronary heart disease in the elderly the three-city cohort study. Arterioscler Thromb Vasc Biol. 2011;31:1445–51.
Wannamethee SG, Whincup PH, Lennon L, et al. Fibrin d-dimer, tissue-type plasminogen activator, von willebrand factor, and risk of incident stroke in older men. Stroke. 2012;43:1206–11.
Carod-Artal FJ, Nunes SV, Portugal D, et al. Ischemic stroke subtypes and thrombophilia in young and elderly Brazilian stroke patients admitted to a rehabilitation hospital. Stroke. 2005;36:2012–4.
Morris JG, Singh S, Fisher M. Testing for inherited thrombophilias in arterial stroke: can it cause more harm than good? Stroke. 2010;41:2985–90.
Whiteley W, Chong WL, Sengupta A, et al. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke. 2009;40:e380–9.
Yeung T, Gilbert GE, Shi J, et al. Membrane phosphatidylserine regulates surface charge and protein localisation. Science. 2008;319:210–3.
Htun P, Fateh-Moghadam S, Tomandl B, et al. Course of platelet activation and platelet-leukocyte interaction in cerebrovascular ischemia. Stroke. 2006;37:2283–7.
Santos-Silva A, Rebelo I, Castro E, et al. Erythrocyte damage and leukocyte activation in ischemic stroke. Clin Chim Acta. 2002;320:29–35.
Owens AP 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res 2011;108:1284–1297.
Jung KH, Chu K, Lee ST, et al. Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann Neurol. 2009;66:191–9.
Simak J, Gelderman MP, Yu H, et al. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006;4:1296–302.
Kuriyama N, Nagakane Y, Hosomi A, et al. Evaluation of factors associated with elevated levels of platelet-derived microparticles in the acute phase of cerebral infarction. Clin Appl Thromb Hemost. 2010;16:26–32.
Chen Y, Xiao Y, Lin Z, et al. The role of circulating platelets microparticles and platelet parameters in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2015;24:2313–20.
Hou J, Fu Y, Zhou J, et al. Lactadherin functions as a probe for phosphatidylserine exposure and as an anticoagulant in the study of stored platelets. Vox Sang. 2011;100:187–95.
Adams Jr HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in amulticenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
Gao C, Xie R, Yu C, et al. Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thromb Haemost. 2012;107:681–9.
Wohner N, Sotonyi P, Machovich R, et al. Lytic resistance of fibrin containing red blood cells. Arterioscler Thromb Vasc Biol. 2011;31:2306–13.
Haapaniemi E, Soinne L, Syrjala M, et al. Serial changes in fibrinolysis and coagulation activation markers in acute and convalescent phase of ischemic stroke. Acta Neurol Scand. 2004;110:242–7.
Liman TG, Bachelier-Walenta K, Neeb L, et al. Circulating endothelial microparticles in female migraineurs with aura. Cephalalgia. 2015;35:88–94.
Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–45.
Sarlon-Bartoli G, Bennis Y, Lacroix R, et al. Plasmatic level of leukocyte-derived microparticles is associated with unstable plaque in asymptomatic patients with high-grade carotid stenosis. J Am Coll Cardiol. 2013;62:1436–41.
Stein ES, Itsekson-Hayosh Z, Aronovich A, et al. Thrombin induces ischemic LTP (iLTP): implications for synaptic plasticity in the acute phase of ischemic stroke. Sci Rep. 2015;5:7912.
Rooth E, Sobocinski-Doliwa P, Antovic J, et al. Thrombin generation in acute cardioembolic and non-cardioembolic ischemic stroke. Scand J Clin Lab Invest. 2013;73:576–84.
Isenegger J, Meier N, Lammle B, et al. D-dimers predict stroke subtype when assessed early. Cerebrovasc Dis. 2010;29:82–6.
Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009;373:155–66.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by grants from the National Natural Science Foundation of China (81270588, 81470301, 81670128, 81670298, and 61575058), Natural Science Foundation of Heilongjiang Province (ZD2015020), and Graduate Innovation Fund of Harbin Medical University (YJSCX2015-56HYD).
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Electronic Supplementary Material
ESM 1
(DOC 39 kb)
Rights and permissions
About this article
Cite this article
Yao, Z., Wang, L., Wu, X. et al. Enhanced Procoagulant Activity on Blood Cells after Acute Ischemic Stroke. Transl. Stroke Res. 8, 83–91 (2017). https://doi.org/10.1007/s12975-016-0501-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-016-0501-7