Abstract
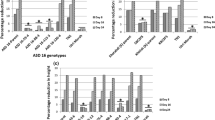
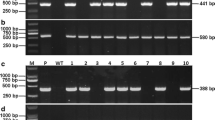
Rice tungro disease (RTD) is one of the most important virus diseases of rice in South and Southeast Asia, which is caused by coinfection of rice tungro bacilliform virus (RTBV) and rice tungro spherical virus (RTSV). RF2a, encoding a bZIP type transcription factor, which is important for rice development is reported to bind to Box II, a crucial cis-element of the RTBV promoter and activate transcription from the RTBV promoter in infected plants. Overexpression of RF2a in transgenic rice had been reported to increase tolerance against the tungro virus. We reported the overexpression of RF2a in transgenic rice plants cultivar IR64. Bioassay using an Indonesian tungro virus isolate indicated that there was a significant increase in the tolerance of the transgenic rice lines compared to the wild-type IR64, and was comparable to the tolerant control Tukad Petanu. qRT-PCR on RF2a-overexpressed transgenic rice and the wild-type control showed that in tungro virus-infected transgenic rice plants in which weak or no RTD symptoms were observed, the relative expression of RF2a were increased. The increase in tolerance toward tungro virus was suggested due to a high level of RF2a in plants that can support rice development amid virus replication. On the contrary, in infected susceptible wild-type plants, in which the level of RF2a was low, severe RTD symptoms were observed. Thus, the availability of RF2a in tungro virus-infected IR64 plants plays an important role in determining the development of RTD symptoms.
Similar content being viewed by others
References
Azzam O, Chancellor TCB. 2002. The biology, epidemiology and management of rice tungro diseases in Asia. Plant Dis. 86(2): 88–100
Bunawan H, Dusik L, Bunawan SR, Amin NM. 2014. Rice tungro disease: From identification to disease control. World Appl. Sci. J. 31(6): 1221–1226
Dahal G, Hibino H, Saxena RC. 1990. Association of leafhopper feeding behavior with transmission of rice tungro to susceptible and tolerant rice cultivar. Phytopathology 80: 371–377
Dai S, Beachy R. 2009. Genetic engineering of rice to resist rice tungro disease. In Vitro Cell. Dev. Biol. Plant 45: 517–524
Dai S, Wei X, Alfonso AA, Pei L, Duque UG, Zhang Z, Babb GM, Beachy RN. 2008. Transgenic rice plants that overexpress transcription factors RF2a and RF2b are tolerant to rice tungro virus replication and disease. Proc. Natl. Acad. Sci. USA 105 (52): 21012–21016
Dai S, Zhang Z, Bick J, Beachy RN. 2006. Essential role of the boxII cis element and cognate host factors in regulating the promoter of rice tungro bacilliform virus. J. Gen. Virol. 87: 715–722
Dai S, Zhang Z, Chen S, Beachy RN. 2004. RF2b, a rice bZIP transcription activator, interacts with RF2a and is involved in symptom development of rice tungro disease. Proc. Natl. Acad. Sci. USA 101(2): 687–692
Dasgupta I, Hull R, Eastop S, Poggi-Pollini C, Blakebrough M, Boulton MI, Davies JW. 1991. Rice tungro bacilliform virus DNA independently infects rice after Agrobacterium-mediated transfer. J. Gen. Virol. 72:1215–1221
Dellaporta SL, Wood J, Hicks BJB. 1983. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1(4):19–21
Doyle JJ, Doyle JL. 1990. Isolation of plant DNA from fresh tissue. Focus 12: 13–15
E ZG, Zhang YP, Zhou JH, Wang L. 2014. Roles of the bZIP gene family in rice. Genet. Mol. Res. 13(2): 3025–3036
Hibino H, Cabauatan PQ. 1987. Infectivity neutralization of rice tungro-associated viruses acquired by vector leafhoppers. Phytopathology 77: 473–476
Hibino H, Daquioag RD, Mesina EM, Aguiero VM. 1990. Tolerances in rice to tungro-associated viruses. Plant Dis. 74: 923–926
Hibino H, Tionco ER, Cabunagan RC, Flores ZM. 1987. Tolerance to rice-tungro associated viruses in rice under experimental and natural conditions. Phytopathology 77: 871–875
Hiei Y, Komari T. 2006. Improved protocols for transformation of indica rice mediated by Agrobacterium tumefaciens. Plant Cell Tiss. Organ Cult. 85(3): 271–283
International Rice Research Institute (IRRI). 2014. Standard Evaluation System (SES) for Rice, 5th Edition. International Rice Research Institute, Manila
Jones MC, Gough K, Dasgupta I, Subba Rao BL, Cliffe J, Qu R, Shen P, Kaniewska M, Blakebrough M, Davies JW, Beachy RN, Hull R. 1991. Rice tungro disease is caused by an RNA and a DNA virus. J. Gen. Viol. 72:757–761
Petruccelli S, Dai S, Carcamo R, Yin Y, Chen S, Beachy RN. 2001. Transcription factor RF2a alters expression of the rice tungro bacilliform virus promoter in transgenic tobacco plants. PNAS 98 (13): 7635–7640
Praptana RH, Sumardiyono YB, Hartono S, Widiarta IN, Muhsin M. 2009. Detection of variability in rice tungro virus from several endemic areas in Indonesia by PCR-RFLP technique (In Indonesian). J. Perlindungan Tanaman Indonesia 15(1): 29–38
Roberts CS, Rajagopal S, Smith LA, Nguyen TA, Yang W, et al. 1997. A comprehensive new set of modular vectors to allow both routine and advanced manipulations and efficient transformation of rice by both Agrobacterium and direct gene-transfer methods. Proceedings of Rockefeller Foundation Meeting of the International Program on Rice Biotechnology, 15–20 September. Malacca, Malaysia
Roy S, Banerjee A, Tarafdar J, Senapati BK. 2012. Detection of probable marker–free transgene-positive rice plants tolerant to rice tungro disease from backcross progenies of transgenic Pusa Basmati1. J. Genet. 91(2): 213–218
Sambrook J, Fritsch E, Maniatis T. 1989. Molecular cloning: A Laboratory Manual 2nd Ed. Cold Spring Harbor Laboratory Press, New York
Sivamani E, Huet H, Shen P, Ong CA, de Kochko A, Fauquet C, Beachy RN. 1999. Rice plant (Oryza sativa L.) containing rice tungro spherical virus (RTSV) coat protein transgenes are tolerant to virus infection. Mol. Breed. 5: 177–185
Sta Cruz FC, Hull R, Azzam O. 2003. Changes in level of virus accumulation and incidence of infection are critical in the characterization of Rice tungro bacilliform virus (RTBV) tolerance in rice. Arch. Virol. 148: 1465–1483
Tyagi H, Rajasubramaniam S, Rajam MV, Dasgupta I. 2008. RNA-interference in rice against Rice tungro bacilliform virus results in its decreased accumulation in inoculated rice plants. Transgenic Res. 17: 897–904
Yin Y, Zhu Q, Dai S, Lamb C, Beachy RN. 1997. RF2a, a bZIP transcriptional activator of the phloem-specific rice tungro bacilliform virus promoter, functions in vascular development. EMBO J 16: 5247–5259
Zhu Q, Ordiz MI, Dabi T, Beachy RN, Lamb C. 2002. Rice TATA binding protein interacts functionally with transcription factor IIB and the RF2a bZip transcriptional activator in an enhanced plant in vitro transcription system. Plant Cell 14: 795–803
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Estiati, A., Astuti, D., Widyajayantie, D. et al. Overexpression of RF2a in Transgenic Rice Plants Cultivar IR64 Enhances Tolerance to Rice Tungro Virus. J. Crop Sci. Biotechnol. 21, 291–299 (2018). https://doi.org/10.1007/s12892-018-0058-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-018-0058-0