Abstract
The single echinoderm microtubule-associated protein-like 4 (EML4) gene and anaplastic lymphoma kinase (ALK) gene fusion is the most common variant of ALK rearrangements in non-small cell lung cancer (NSCLC). Herein, we firstly report that coexistence of a novel histone methyltransferase (SETD2)-ALK, EML4-ALK double-fusion is sensitive to alectinib as first-line therapy, and response to immunotherapy combined with chemotherapy after resistant. The patient responded to alectinib as a first-line therapy and achieved progression-free survival (PFS) for 26 months. After resistance, liquid biopsy showed that the reason of drug resistance was the disappearance of SETD2-ALK and EML4-ALK fusion variants. In addition, chemotherapy combined with immunotherapy subsequently achieved a survival benefit of more than 25 months. Therefore, alectinib may be a viable therapeutic option for NSCLC patients with double ALK fusion and immunotherapy combined with chemotherapy may be a viable therapeutic option when double ALK fusion loss may be the mechanism of alectinib resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The anaplastic lymphoma kinase (ALK) gene fusion accounts for about 3–6% of non-small cell lung cancer (NSCLC), which is the second identified targetable driver gene followed by epidermal growth factor receptor (EGFR) mutations in NSCLC [1]. It usually occurs in young, nonsmoking patients with lung adenocarcinoma [2]. At present, according to the National Comprehensive Cancer Network (NCCN) guidelines, the second-generation ALK-tyrosine kinase inhibitors (TKIs), alectinib and brigatinib, and the third-generation ALK-TKI, lorlatinib, are the preferred first-line treatment recommendations for advanced NSCLC patients with ALK gene rearrangements [3]. In clinical practices, patients who simultaneously undergo double ALK fusion are rare, and the fused genes are also diverse [4,5,6,7,8,9,10]. In this report, we present for the first time an unreported case of lung adenocarcinoma with double ALK fusions of echinoderm microtubule-associated protein like 4 (EML4) and histone methyltransferase (SETD2), which is sensitive to alectinib. When the drug resistance to AKI-TKIs occurred, the next-generation sequencing (NGS) test showed that double ALK fusions disappeared. Subsequent immunotherapy combined with chemotherapy is effective and achieves long-term survival benefit.
2 Case presentation
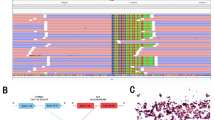
A 28-year-old male with no smoking history presented to the hospital with a persistent cough for half a month in August 2018. Chest computed tomography (CT) scan revealed left upper lobe bronchial stenosis, enlarged multiple mediastinal (2R, 5, 6, 7 zones) and left hilar lymph nodes with multiple nodules in both lungs, left pleural thickening with bilateral pleural and pericardial effusions. And multiple nodular high-density shadows were seen in the left 3rd and 8th ribs, sternum, C1, 7, 11, 12, and the left adnexa of C1. Central type carcinoma of left lung with intrapulmonary, pleural and bone metastasis was considered. Endoscopic biopsy of the left pleural tissue suggested lung adenocarcinoma with ALK-Ventana D5F3, keratin 7, napsin A and thyroid transcription factor-1 (TTF-1) positivity, and adenocarcinoma cells were also found in pleural effusion and pericardial effusion. And EML4-ALK (E14:A21, allelic frequency: 14.05%) and SETD2-ALK (S12:A21, allelic frequency: 25.20%) double-fusion (Fig. 1) were found in the pleural tissue by DNA-based NGS, which can capture all exons of 31 genes that are highly related to the occurrence and development of cancer, and important exons and partial introns of 137 genes that are related to individualized treatment or high frequency mutation of lung cancer, and perform high-depth sequencing up to 1000X. (Burning Rock, Guangzhou, China). His disease was diagnosed as left lung adenocarcinoma with left hilar and bilateral mediastinal lymph nodes, bilateral lungs, pleura and bone metastasis (cT4N3M1c, stage IVB).
The National Medical Products Administration of China (NMPA) approved alectinib for ALK-positive patients with locally advanced or metastatic NSCLC in 2018. The patient began targeted therapy with alectinib (600 mg twice daily) in October 2018. During the period of regular reexamination, the efficacy evaluation was stable disease (SD). After 26 months of treatment, the patient developed chest tightness and shortness of breath again, and chest CT showed bilateral pleural effusion and pericardial effusion again. At the same time, the lesion was enlarged than before, and a large amount of abdominal effusion appeared. No obvious tumor evidence was found in pathological examination of pleural effusion and ascites. According to the patient’s CT and condition, alectinib resistance and disease progression were considered. NGS detection was performed in pleural effusion, abdominal effusion, pericardial effusion and peripheral blood respectively, and no mutation was detected. Given the progress of the disease, pemetrexed plus carboplatin combined with toripalimab was used for six cycles from January 2021, and then maintenance treatment with toripalimab rather than pemetrexed combined toripalimab due to intolerance side effects and willingness of patient. The pulmonary lesion was slightly enlarged again after 16 months of treatment. Considering that pemetrexed had achieved good effect in the past, pemetrexed was added again. After 6 cycles of pemetrexed combined with toripalimab treatment, toripalimab maintenance treatment was performed again. At the deadline of follow-up, the patient continued to receive maintenance treatment with toripalimab. (Fig. 2)
(A) Pathological picture of adenocarcinoma. (B) CT shows disease progression after 26 months of targeted therapy with aletinib; The left interlobar fissure focus and mediastinal lymph node enlargement appeared, together with pleural effusion and new massive intraperitoneal effusion. (C) CT shows that the disease was stable after 6-cycle chemotherapy combined with immunotherapy. (D) CT shows that the left lung lesion and mediastinal lymph node were larger than before after 12 months of immune monotherapy. (E) CT shows that the lesion was stable after another 6-cycle treatment with chemotherapy and immunotherapy.
P, pemetrexed; C, carboplatin; T, Toripalimab; PD, Progressive disease; SD, stable disease.
3 Discussion
ALK mutations are most commonly caused by fusion with EML4 [11]. Approximately 61–74% of patients with EML4-ALK positive responded to ALK-TKIs. There are more than 15 different EML4-ALK fusion variants reported in NSCLC. In which V1 (E13:A20), V2 (E20:A20) and V3a/b (E6a/b:A20) are the most common. A previous retrospective study has found that variant V1/2 is more sensitive to ALK-TKIs than variant V3a/b, suggesting that different EML4-ALK variants may show different sensitivity to ALK-TKIs [12,13,14]. However, the sensitivity of fusion of “E14:A21” in this case has not been previously reported.
SETD2-ALK gene fusion is a rare ALK fusion, and it has been rarely reported in the previous studies. J. Kang et al. found that SETD2-ALK gene fusion at the DNA level expressed the EML4-ALK transcripts during mRNA maturation. It plays a role of “mediation”. In addition, the study also found that there was no significant difference in overall survival (OS) between different EML4-ALK fusion subtypes (P = 0.386), while multiple ALK fusion was significantly higher than that of EML4-ALK fusion (P = 0.01). It is possible that tumors with multiple ALK fusions are likely to be more addicted to the ALK signaling pathway, thus making the ALK-TKIs have more profound effects [14]. However, in this case, the patient with EML4-ALK and SETD2-ALK double-fusion had a progression-free survival (PFS) of 26 months after targeted therapy with alectinib, which was similar to the median PFS of 25.7 months assessed by an independent evaluator in the ALEX trial [15]. Therefore, NSCLC patients with double or compound mutations still can have response and survival benefit to targeted therapies. SETD2-ALK can be a rare fusion mutation which is sensitive to the treatment of ALK inhibitors.
Individualized treatment should be given according to the mechanism of ALK-TKIs resistance. 56% of patients with ALK gene fusion mutations developed ALK resistance mutations after the use of second-generation ALK inhibitors, with ALK G1202R becoming the most common ALK resistance mutation. The third generation ALK-TKIs, lorlatinib, can effectively inhibit the ALK resistance mutation. But in the remaining 44%, the mutation disappeared after the use of second-generation ALK inhibitors [16]. The ALUR study showed that for patients who failed to detect ALK fusion after first-line crizotinib resistance, compared with patients with ALK fusion after treatment, the objective response rate (ORR) of second-line treatment with alectinib was lower, and there was no significant improvement compared with chemotherapy. The results suggest that it may be more reasonable to choose the treatment mode of chemotherapy when ALK fusion is not detected after ALK-TKIs resistance [17]. Furthermore, a retrospective study including 20 patients with ALK fusion and disease progression after first-line aletinib treatment showed that the curative effect of ALK-TKIs in patients with secondary mutation of ALK is better than that in patients without secondary mutation of ALK (PFS: 242d vs. 75d, P = 0.05, HR = 0.46, 95% CI: 0.18–1.2). For patients without mutation, most of the them received at least first-line chemotherapy, and it was found that chemotherapy achieved better PFS (168d vs. 75d, P = 0.035, HR = 0.47, 95% CI: 0.19–1.2) than ALK-TKIs [18]. Therefore, the benefit of using ALK-TKIs is limited for patients who have ALK fusion before treatment but whose ALK fusion disappears after treatment, and the benefit may be more obvious when chemotherapy is used for subsequent treatment compared with targeted therapy.
In conclusion, this is the first report of an advanced and metastatic lung adenocarcinoma patient harbored EML4-ALK and SETD2-ALK double-fusion variation and successively response to alectinib and immunotherapy combined with chemotherapy after alectinib resistant. Loss of both EML4-ALK and SETD2-ALK double-fusion may be the mechanism of aletinib resistance for this patient. Immunotherapy combined with chemotherapy as second-line treatment may be a viable therapeutic option for NSCLC patients with double-ALK fusion when loss of double-fusion is the mechanism of ALK-TKIs resistance. However, the study also has certain limitations that must be acknowledged. During the progress of targeted treatment with aletinib, cancer cells were not found in pleural effusion, peritoneal effusion and pericardial effusion, so NSG detection of serous effusion may be negative. At the same time, the detection rate of plasma NGS detection was lower than tissues, which may affect the accuracy of NGS detection results. The consideration of ALK gene loss was more from our understanding of the disease, and there was no definite basis for it. Readers should interpret it with caution.
Data availability
All data generated or analysed during this study are included in this published article.
References
Yu Y, et al. Frequencies of ALK rearrangements in lung adenocarcinoma subtypes: a study of 2299 chinese cases. Springerplus. 2016;5(1):894.
Hallberg B, Palmer RH. The role of the ALK receptor in cancer biology. Ann Oncol. 2016;27(Suppl 3):iii1–15.
Non-Small Cell Lung Cancer, Version 5 NCCN Clinical Practice Guidelines in Oncology. 2022.
Liang Q, et al. Coexistence of a novel NBEA-ALK, EML4-ALK double-fusion in a lung adenocarcinoma patient and response to alectinib: a case report. Lung Cancer. 2021;162:86–9.
Luo J, et al. Coexistence of a Novel PRKCB-ALK, EML4-ALK Double-Fusion in a lung adenocarcinoma patient and response to Crizotinib. J Thorac Oncol. 2019;14(12):e266–8.
Tao H, et al. Concomitant novel ALK-SSH2, EML4-ALK and ARID2-ALK, EML4-ALK double-fusion variants and confer sensitivity to crizotinib in two lung adenocarcinoma patients, respectively. Diagn Pathol. 2022;17(1):27.
Zhong JM, et al. A novel EML4-ALK BIRC6-ALK double fusion variant in lung adenocarcinoma confers sensitivity to alectinib. Lung Cancer. 2020;145:211–2.
Wu X, et al. Novel NLRC4-ALK and EML4-ALK double fusion mutations in a lung adenocarcinoma patient: a case report. Thorac Cancer. 2020;11(6):1695–8.
Zeng H, et al. Case Report: identification of two rare fusions, PDK1-ALK and STRN-ALK, that coexist in a lung adenocarcinoma patient and the response to Alectinib. Front Oncol. 2021;11:722843.
Qin BD, et al. Identification of a novel EML4-ALK, BCL11A-ALK double-fusion variant in lung adenocarcinoma using next-generation sequencing and response to crizotinib. J Thorac Oncol. 2019;14(6):e115-7.
Gridelli C, et al. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev. 2014;40(2):300–6.
Sabir SR, et al. EML4-ALK Variants: Biological and molecular properties, and the implications for patients. Cancers. 2017;9(9):118.
Woo CG, et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann Oncol. 2017;28(4):791–7.
Kang J, et al. Complex ALK fusions are associated with better prognosis in advanced non-small cell lung cancer. Front Oncol. 2020;10:596937.
Peters S, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–38.
Gainor JF, et al. Molecular mechanisms of resistance to First- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118–33.
Wolf J, et al. OA02.07 phase 3 ALUR Study of Alectinib in pretreated ALK + NSCLC: final efficacy, safety and targeted genomic sequencing analyses. J Thorac Oncol. 2019;14(10):S210.
Zou Z, et al. EP08.02-009 progression pattern, resistance mechanism and subsequent therapy for ALK positive NSCLC in the era of seconD-generation ALK-TKIs. J Thorac Oncol. 2022;17(9):S400.
Acknowledgements
The authors thank the patient and his family.
Funding
This work was supported by National Natural Science Foundation of China (No.81903981), Zhejiang Provincial Natural Science Foundation of China (No. LY21H290002).
Author information
Authors and Affiliations
Contributions
LZ: Visualization, Validation, Methodology, Writing—original draft preparation JQ: Writing- Reviewing and Editing, Funding acquisition, Supervision, Conceptualization, Project administration. Both authors have read and agreed to the published version of the manuscript. Both authors read and approved the final maunscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Ethical Committee of Zhejiang Cancer Hospital and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
The included patient gave his oral and written informed consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, L., Qin, J. Coexistence of a novel SETD2-ALK, EML4-ALK double-fusion in an advanced lung adenocarcinoma patient after alectinib resistant and response to immunotherapy combined with chemotherapy: a case report. Discov Onc 14, 44 (2023). https://doi.org/10.1007/s12672-023-00654-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00654-x