Abstract
Gastric cancer perforation is a life-threatening condition that accounts for less than 5% of all gastric cancer patients and typically requires emergency surgery. However, preoperative diagnosis is difficult and management has a dual purpose: to treat peritonitis and to achieve a curative resection. The optimal surgical strategy is still unclear and prognosis remains poor. A search of the literature was performed using MEDLINE databases (Pubmed, EMBASE, Web of Science and Cochrane) using terms such as “perforated gastric cancer”, “perforated gastric cancer and surgery”, “perforated gastric tumour” and “gastric cancer perforated”. Case reports, other reviews, non-english written papers and papers written before 2010 were excluded. Eight articles published between 2010 and 2020 matched the inclusion criteria for this review. Perforated gastric cancer was more prevalent in elderly males. Distal stomach was most frequently involved. Preoperative diagnosis was uncommon. Mortality rates ranged from 2 to 46%. Patients able to receive an R0 resection demonstrated better long-term survival compared with patients who had simple closure procedures. Laparoscopic procedure was mentioned only in one study. In an emergency situation, curative RO resection should always be offered in patients without multiple adverse factors. A surgical strategy using laparoscopic local repair as first step of surgery to resolve the peritonitis followed by a radical open or laparoscopic gastrectomy with lymphadenectomy could be considered. A balance between emergency and oncological needs should drive the surgical choice on a case by case basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gastric cancer (GC) is one of the leading causes of death worldwide although its incidence has been declined [1, 2]. The spontaneous perforation of gastric cancer (PGC) is a life-threatening condition that occurs in less than 5% of all gastric cancer patients and it is a surgical emergency fraught with numerous challenges. In most circumstances, PGC is not Known preoperatively and is associated to advanced disease stage [3]. It is difficult to preoperatively diagnose PGC because its symptoms are the same as those of a perforated gastric ulcer. Patients experiencing gastric perforation exhibit acute onset abdominal pain with evidence of free air on plain abdominal X-ray. Furthermore, it may be difficult to distinguish a gastric cancer from a gastric ulcer at the time of surgery unless a pre-operative diagnosis of GC is known or a clear metastatic disease is found at laparotomy. Therefore, diagnosis of malignancy is made post-operatively in most of the cases [4, 5]. An intraoperative frozen section could help in the matter. When diffuse peritonitis is diagnosed, emergent surgery is necessary. The aims of surgery in these patients are two-fold: to resolve peritonitis and to achieve a curative resection. The ideal option in treating the malignancy is unclear and it may be a result of various factors such as those related to the emergency presentation (hemodynamic stability of the patient, extent of peritonitis, active comorbidities) and those depending on the surgical expertise and the stage of the malignancy [6, 7]. The surgical procedures range from a simple lavage and perforation closure to resection that can be performed by employing the one-stage or two stage technique. The first procedure treats life-threatening peritonitis followed by the second procedure which includes definitive gastrectomy with appropriate lymphadenectomy [4, 5]. The short-term outcome in these patients is often poor due to the septic complications following the peritonitis and to the post-operative morbidity. Moreover, also the long-term outcome may be poor due to the advanced stage of cancer and the early development of peritoneal metastases related to the perforation [8].
The optimal surgical strategy for PGC is still debated. This paper aims to review the surgical options in case of perforated gastric cancer and focuses on surgical outcomes, survival rates and pre-operative diagnosis.
1.1 Search strategy
An electronic search was conducted using MEDLINE databases (Pubmed, EMBASE, Cochrane and Web of Science) matching terms such as “perforated gastric cancer”, “perforated gastric cancer and surgery”, “perforated gastric tumor” and “gastric cancer perforated”. All publications in English between 2010 and 2020 were reviewed. Case reports, reviews, meta-analyses, abstracts, non-english papers and letters written before 2010 were excluded. Cases describing iatrogenic gastric perforation related to endoscopic mucosal resection (EMR) or endoscopic submucosal resection (ESD) for early gastric cancer were not included. Pathological gastric perforations caused by gastric lymphoma and metastatic melanoma or lung cancer were not considered. The following data were retrieved from the publications: author, publication year, country, number of patients, gender, age, tumor location, tumor stage, pre-operative diagnosis of gastric cancer, type of emergency surgery (repair versus resection), type of gastrectomy (one-stage versus two stage), use of laparoscopic procedure, R0 resections, post-operative morbidity and mortality and survival time. A statistical analysis comparing survival curves for each surgical procedure have been conducted by mean of log rank test; a p-value < 0.05 has been considered significant.
1.2 Patients characteristics
Eight studies published between 2010 and 2020 matched the inclusion criteria in this review. The preoperative characteristics of the patient population are shown in Table 1. There is a great heterogeneity in patients characteristics due to the wide difference in the consistency of sample which range from 8 to 2964 patients. Patients age ranged from 60 to 79 years (mean 70 year) and they were predominantly males. Data on preoperative diagnosis of gastric cancer were reported only in five studies, and account for a mean of 38% of the patients [5, 7, 9,10,11]. The most common tumor location was the distal part of the stomach in 37% of the patients, followed by the middle third in 36% and the upper third in 27%. The majority of the patients (66%) presented stage III or IV disease.
1.3 Surgical procedures and outcomes
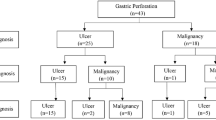
A total of 476 patients underwent total or subtotal gastrectomy as a one-stage or staged operation according to their general conditions. Only one study mentioned a laparoscopic approach in 38 patients who underwent laparoscopic gastrectomy performed by an experienced surgeon [5]. Moreover, 140 patients underwent repair with simple closure or omental patch and 12 patients were treated non-surgically due to poor conditions or refusal. 441 patients had single-stage gastrectomy and 55 had only damage-control surgery or an initial conservative management followed by gastrectomy (Table 2). Poor overall general conditions, high clinical risk or advanced disease were reasons provided for simple closure. Two-stage operations for improved oncological outcome were discussed in three studies [10,11,12]. Wang et al. [9] described a scheduled gastrectomy following a primary closure in 4 patients according to clinical conditions. Hata et al. [12] reported their experience on 514 patients. Of them, 388 patients underwent gastrectomy, completed as one stage in 376 and in two-stage in the remnant 12. Of the 114 cases treated by performing simple closure or omental patch, 38 received a second surgery: gastrectomy was performed in 32 but not in the remaining 6 cases because of advanced stage (Fig. 1). 12 more patients were treated conservatively due to limited peritonitis and subsequently 10 of them received gastrectomy and were included in the group of patients who underwent two stage procedure. Ignjatovic et al. [10], out of 11 patients treated, performed one-stage procedure in 3 (27.2%) and two-stage approach in 5 (45.6%). Simple closure with omental patch was performed in the other 3 patients. Post-operative morbidity was reported only in one study and accounted for 35% [5]. Overall operative mortality rates changed very widely throughout all the studies, ranging from 2 to 46% (mean 18%). As a common sense, usually surgeon skills did not influence the surgical choice. Indeed, the laparoscopic approach can be performed only in selected referral centers where an advanced expertise in laparoscopic gastric surgery is available. Data regarding number and type of surgical procedures are reported in Fig. 2.
1.4 Prognostic factors and survival
Different survival outcomes were also reported among the studies. As expected, R0 resection was always associated to improved survival if compared to R1/2 or palliative procedures. Data regarding median survival for each procedure are shown in Fig. 2. Differences between survival curves showed statistical significance (p < 0.001—log-rank test). Kim et al. [5] performed radical gastrectomy with D2 lymphadenectomy in 35 patients and reported an overall 5-year survival of 19.5%. The mean survival time was 23 months ranging from 2 months for patients who underwent palliative surgery to 75 months for patients who underwent curative resection. Only three papers report data about adjuvant chemotherapy but none points out differences in survival between patients who underwent adjuvant chemotherapy and those who did not [5, 7, 15].
2 Discussion
Perforated gastric cancer (PGC) accounts for less than 1% of cases of acute abdomen but it represents the main oncologic emergency after major bleeding [13]. Although gastric cancer is the third leading cause of death from cancer worldwide [14], the management of PGC is still debated due to the lack of clinical guidelines supporting a specific algorithm in an emergency situation where surgery has a dual purpose: treating life-threatening peritonitis and curing gastric cancer. Even if gastric cancer can be diagnosed preoperatively or intra-operatively, the choice about treatment in PGC depends on several factors regarding emergency, oncologic and patient variables, such as severity of peritonitis, hemodynamic instability, sepsis, presence of comorbidities, presence of metastases at exploration [8].
In this review, only 38% of the patients had a pre-operative diagnosis of gastric cancer and this is in line with data reported in other reviews on the topic [4, 8]. PGC is a rare condition that accounts for less than 5% of all gastric cancers [8, 10] but it is very difficult to diagnose before and at surgery and the treatment goal, balancing oncologic with emergency criteria, still represents a challenging issue. Given that patients with benign and malignant gastric perforation exhibit similar symptoms such as generalized abdominal pain, rebound tenderness, poor systemic conditions caused by peritonitis that make a detailed clinical examination difficult, the diagnosis of gastric cancer is frequently made post-operatively. Moreover, intraoperative endoscopy and frozen section are often not available in emergency; furthermore, inflammatory changes associated with peritonitis resemble those caused by tumor invasion leading to misinterpretation and overestimation of intraoperative findings [9, 13]. In this review, 66% of patients had stage III-IV disease, and the overall mortality ranged from 2 to 46%. In the past, primary closure was the surgical treatment of perforation because PGC was thought to indicate terminal disease. In some cases, leakage at the suture site required a second operation after which the patient’s general conditions worsened [7]. Since then, several reports on PGC have shown a significantly better prognosis for patients who underwent curative resection if compared to those who underwent non-curative resection [4, 5, 7, 9, 12]. Two surgical methods are used in management of PGC. Single stage radical D2 gastrectomy is recommended if the patient’s general conditions are favorable. Hata et al. [12], in their study, found a statistically significant difference in operative mortality between one-stage and two-stage gastrectomy patients (11.4% versus 1.9%). In the same series, a curative resection (R0) was achieved in 50% of the one-stage gastrectomy group and in 78.4% of the two-stage gastrectomy group. Regardless of whether patients underwent a one-stage or two-stage gastrectomy, curative R0 resection improved survival. This indicated that when curative R0 resection cannot be immediately performed due to peritonitis, palliative or non-curative gastrectomy should be avoided and two-stage gastrectomy should be planned following peritonitis recovery. In the same study, Hata et al. [12] described a median survival of 75 months and a 5-year survival rate of about 50% when a curative resection was possible. Similar results were obtained by Ignjatovic et al. [10] who demonstrated a higher survival rate among patients who underwent curative resection if compared to those who underwent simple closure with omental patch (75.77 days vs 18 days). Also the oncologic value of the surgical procedure seems to be worsened by the emergency setting of these patients: Fisher et al. [15] in their paper found that patients who underwent urgent surgery for gastric cancer had significantly worse quality resections with decreased lymph node retrieved and increased positive margins as well as increased 30 day and overall mortality if compared to patients undergoing elective surgery.
In regards to the recurrence rate after PGC, Tsujimoto et al. [13] showed that there were no significant differences between perforated and non-perforated gastric cancer. Mahar et al. [4] also showed that peritoneal contamination due to perforation did not affect survival as it was thought in the past. According to Tan et al. [7] long-term survival was dependent on the stage of malignancy. As demonstrated in his small series advanced stage of the gastric malignancy and the possibility of tumor seeding of the peritoneal cavity led to unfavorable outcomes. According to these results, we could conclude that emergency presentation, regardless to the severity of the clinical findings, would not affect survival except for its negative impact on quality of oncologic resection.
Nowadays, minimally invasive surgery can be considered a suitable option for perforated gastric or duodenal ulcer, both for diagnosis or elective resection [16,17,18]. Laparoscopic gastrectomy appeared comparable with open technique in terms of overall and disease-free survival [19, 20]. On the other hand, laparoscopic gastrectomy in emergency cannot be considered a standard choice so far. Kim et al. [5] reported a laparoscopic gastrectomy with D2 lymphadenectomy performed in 35 of 43 patients with PGC. In their high specialized experience primary laparoscopic gastrectomy was recommended in emergency only when an appropriate expertise is available. Indeed, laparoscopic primary gastrectomy with lymphadenectomy could be the most promising treatment for PGC if the patient general conditions and surgeon ability allow it but it cannot be still considered the standard of care.
In conclusion, the most important goal for PGC is to achieve curative R0 resection, regardless of whether the surgical approach is a one-stage or two-stage gastrectomy. When gastric cancer can be diagnosed before or at surgery, one-stage gastrectomy should be performed in those cases with limited peritonitis and when a curative R0 resection can be achieved. On the contrary, if curative R0 resection cannot be achieved due to diffuse peritonitis, it is important to treat the peritonitis first and then to plan a two-stage gastrectomy. Whether the procedure should be performed laparoscopically or open depends on the surgical expertise and on the patient’s general conditions. A laparoscopic approach to treat the peritonitis and the perforation followed by a curative surgery could definitely reduce intra-abdominal adhesions and facilitate the staged gastrectomy. Future studies should evaluate how to improve the preoperative diagnosis as well as the more appropriate surgical choice, also considering the promising results offered by neoadjuvant chemotherapy and the still debated indications of the intraoperative chemotherapy in order to reduce the cancer cell seeding and increase long-term survival. Also, the prognostic role of adjuvant treatments in this subset of gastric cancer patients should be better assessed: only three papers reported data about adjuvant chemotherapy delivery but none pointed out its role in conditioning survival and no statistical differences have been reported between patients undergoing it or not. Since gastric cancer has the highest rate of peritoneal metastasis, prevention of peritoneal carcinomatosis is a critical need. In selected cases of PGC, laparoscopic surgery associated with hyperthermic intraoperative chemotherapy (HIPEC) has shown some benefit in reducing the risk of peritoneal recurrence [21, 22] although the real mechanisms regulating peritoneal seeding of tumor cells from GI cancers still need to be better clarified [23,24,25,26].
As a general recommendation, we can conclude that the best results in terms of surgical outcome and overall survival are obtained when treatment strategy is tailored on general conditions of patients, especially in regards to the extent of peritonitis; a balance between oncologic and emergency criteria should be the guiding light for the treatment choice on a case-by-case basis. One stage or staged gastrectomy with curative intent (i.e. oncological criteria) should always be preferred if technically feasible when a gastric tumor is suspected even when a definitive histology is not provided. Simple closure or omental patch can be considered valuable options instead of gastrectomy when life-threatening conditions are prevalent; similar results in terms of oncologic outcome are reported for these patients if radical surgery is postponed and neoadjuvant and/or adjuvant chemotherapy are administered. Indications for laparoscopic surgery remain limited.
This study has some limitations due to the heterogeneity of the samples, the rarity of PGC, the lack of the histopathology among the studies, the lack of a homogeneous assessment of clinical conditions that have led to a surgical approach instead than another. Future studies should standardize a proper algorithm to deal with this life-threatening condition in order to ameliorate the overall survival of these patients.
Data availability
Data are available upon request.
References
Casamayor M, Morlock R, Maeda H, Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience. 2018;12:883.
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736–88.
Vasas P, Wiggins T, Chaudry A, Bryant C, Hughes FS. Emergency presentation of the gastric cancer; prognosis and implications for service planning. World J Emerg Surg. 2012;7(1):31.
Mahar AL, Brar SS, Coburn NG, Law C, Helyer LK. Surgical management of gastric perforation in the setting of gastric cancer. Gastric Cancer. 2012;15:S146–52.
Kim HS, Lee JH, Kim MG. Outcomes of laparoscopic primary gastrectomy with curative intent for gastric perforation: experience from a single surgeon. Surg Endosc. 2020. https://doi.org/10.1007/s00464-020-07902-z.
Kandel BP, Singh Y, Singh KP, Khakurel M. Gastric cancer perforation: Experience from a tertiary care hospital. J Nepal Med Assoc. 2013;52(191):489–93.
Tan KK, Quek TJL, Wong N, Li KK, Lim KH. Emergency surgery for perforated gastric malignancy: an institution’s experience and review of the literature. J Gastrointest Oncol . 2011;2(1):13–8.
Melloni M, Bernardi D, Asti E, Bonavina L. Perforated gastric cancer: a systematic review. J Laparoendosc Adv Surg Tech A. 2020;30(2):156–62.
Wang SY, Hsu CH, Liao CH, Fu CY, Ouyang CH, Cheng CT, Hsu JT, Yeh TS, Yeh CN. Surgical Outcome evaluation of perforated gastric cancer: from the aspects of both acute care surgery and surgical oncology. Scand J Gastroenterol. 2017;52:1371–6.
Ignjatovic N, Stojanov D, Djordjevic M, Ignjatovic J, Stojanov DB, Milojkovic B. Perforation of gastric cancer-what should the surgeon do? Bosn J Basic Med Sci. 2016;16:222–6.
Kim BS, Oh ST, Yook JH, Kim BS. What is the appropriate management for perforated gastric cancer? Am Surg. 2014;80:517–20.
Hata T, Sakata N, Kudoh K, Shibata C, Unno M. The best surgical approach for perforated gastric cancer: one-stage vs two-stage gastrectomy. Gastric Cancer. 2014;17:578–87.
Tsujimoto H, Hiraki S, Sakamoto N, Yaguchi Y, Horio T, Kumano I, Akase T, Sugasawa H, Aiko S, Ono S, Ichikura T, Kazuo H. Outcome after emergency surgery in patients with a free perforation caused by gastric cancer. Exp Ther Med. 2010;1:199–203.
Globocan. World Health Organization. Global Health Observatory. Geneva: World Health Organization; 2018. p. 2018.
Fisher BW, Fluck M, Young K, Shabahang M, Blansfield J, Arora TK. Urgent Surgery for gastric adenocarcinoma: a study of the national Cancer Database. J Surg Res. 2020;245:619–28.
Soreide K, Thorsen K, Soreide JA. Strategies to improve the outcome of emergency surgery for perforated peptic ulcer. Br J Surg. 2014;101:e51–64.
Irino T, Sano T, Hiki N, et al. Diagnostic staging laparoscopy in gastric cancer: a prospective cohort at a cancer institute in Japan. Surg Endosc. 2018;32:268–75.
Sica GS, Djapardy V, Westaby S, Maynard ND. Diagnosis and management of aortoesophageal fistula caused by a foreign body. Ann Thoracic Surg. 2004;77(6):2217–8. https://doi.org/10.1016/j.athoracsur.2003.06.031.
Sica GS, Iaculli E, Biancone L, Di Carlo S, Scaramuzzo R, Fiorani C, Gentileschi P, Gaspari AL. Comparative study of laparoscopic vs open gastrectomy in gastric cancer management. WJG. 2011. https://doi.org/10.3748/wjg.v17.i41.4602.
Quan Y, Huang A, Ye M, et al. Comparison of laparoscopic versus open gastrectomy for advanced gastric cancer: an updated meta-analysis. Gastric Cancer. 2016;19:939–50.
Badgwell B, Blum M, Das P, et al. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann Surg Oncol. 2017;24:3338–44.
Bernardi D, Asti E, Punturieri M, et al. Laparoscopic gastrectomy and adjuvant hypertermic intraperitoneal chemotherapy (HIPEC) using a closed system with turbulent-flow circuit: technical aspects and preliminary results of a pilot study. Eur Surg. 2018;50:209–14.
Franzè E, Marafini I, De Simone V, et al. Interleukin-34 induces Cc-chemokine Ligand 20 in gut epithelial cells. J Crohn Colitis. 2016;10(1):87–94. https://doi.org/10.1093/ecco-jcc/jjv181.
EuroSurg Collaborative. EuroSurg: a new European student-driven research network in surgery. Colorectal Dis. 2016;18(2):214–5. https://doi.org/10.1111/codi.13260.
Franzè E, Dinallo V, Rizzo A, et al. Interleukin-34 sustains pro-tumorigenic signals in colon cancer tissue. Oncotarget. 2017;9(3):3432–45. https://doi.org/10.18632/oncotarget.23289.
Sica GS, Fiorani C, Stolfi C, Monteleone G, Candi E, Amelio I, Catani V, Sibio S, Divizia A, Tema G, Iaculli E, Gaspari AL. Peritoneal expression of Matrilysin helps identify early post-operative recurrence of colorectal cancer. Oncotarget. 2015;6(15):13402–15. https://doi.org/10.18632/oncotarget.2830.
Funding
No financial support was obtained for this paper.
Author information
Authors and Affiliations
Contributions
SDCand MF wrote the manuscript. GC and DV reviewed data and literature. MC and PR revised the draft. SS revised the final version of the manuscript.All authors made substantial contributions to the conception or design of the work, the acquisition, analysis, and interpretation of data; all revised it critically for important intellectual content and approved the version to be published; they are all agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Carlo, S., Franceschilli, M., Rossi, P. et al. Perforated gastric cancer: a critical appraisal. Discov Onc 12, 15 (2021). https://doi.org/10.1007/s12672-021-00410-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-021-00410-z