Abstract
Higher levels of circulating estrogens and estrogen metabolites (EMs) have been associated with higher breast cancer risk. In breast tissues, reduced levels of terminal duct lobular unit (TDLU) involution, as reflected by higher numbers of TDLUs and acini per TDLU, have also been linked to elevated breast cancer risk. However, it is unknown whether reduced TDLU involution mediates the risk associated with circulating EMs. In a cross-sectional analysis of 94 premenopausal and 92 postmenopausal women referred for clinical breast biopsy at an academic facility in Vermont, we examined the associations of 15 EMs, quantified using liquid chromatography-tandem mass spectrometry, with the number of TDLUs and acini count/TDLU using zero-inflated Poisson regression with a robust variance estimator and ordinal logistic regression models, respectively. All analyses were stratified by menopausal status and adjusted for potential confounders. Among premenopausal women, comparing the highest vs. the lowest tertiles, levels of unconjugated estradiol (risk ratio (RR) = 1.74, 95 % confidence interval (CI) = 1.06–2.87, p trend = 0.03), 2-hydroxyestrone (RR = 1.74, 95 % CI = 1.01–3.01, p trend = 0.04), and 4-hydroxyestrone (RR = 1.74, 95 % CI = 0.99–3.06, p trend = 0.04) were associated with significantly higher TDLU count. Among postmenopausal women, higher levels of estradiol (RR = 2.09, 95 % CI = 1.01–4.30, p trend = 0.04) and 16α-hydroxyestrone (RR = 2.27, 95 % CI = 1.29–3.99, p trend = 0.02) were significantly associated with higher TDLU count. Among postmenopausal women, higher levels of EMs, specifically conjugated estrone and 2- and 4-pathway catechols, were also associated with higher acini count/TDLU. Our data suggest that higher levels of serum EMs are generally associated with lower levels of TDLU involution.
Similar content being viewed by others
Introduction
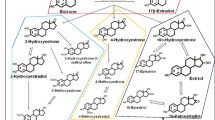
High levels of exposure to endogenous estrogens have been shown to increase breast cancer risk [1, 2]. Pooled analyses of prospective studies have estimated a 1.4-fold higher premenopausal breast cancer risk [1] and a twofold higher postmenopausal breast cancer risk [2] in women with the highest vs. the lowest quintiles of circulating estradiol levels. With advances in technology that have allowed reliable measurements of individual estrogen metabolites (EMs), recent studies have found similar positive associations between serum EMs and postmenopausal breast cancer risk [3–5]. Estrogen metabolites are formed when parent estrogens (estrone, estradiol) are hydroxylated at the 2-, 4-, or 16-carbon positions of the steroid ring and are hypothesized to stimulate cell proliferation largely through estrogen receptor (ER)-mediated mechanisms and to damage DNA through producing quinone DNA adducts [6–8]. The individual EMs have varying degrees of carcinogenic potential depending on their hydroxylation pathway, methylation, and conjugation status [9–11]. For example, 2-pathway EMs have been suggested to have lower estrogenic potential as they have a faster rate of dissociation from ER than 4-pathway EMs [12]. Methylated catechols of 2- and 4-pathways are hypothesized to be less genotoxic than catechols as they do not undergo further redox cycling [12, 13].
Terminal duct lobular units (TDLUs) are the predominant anatomical structures of the breast from which breast carcinomas originate [14]. As women age, the numbers of TDLUs and acini (epithelial substructures) within TDLUs decrease through a process called TDLU involution [15]. Reduced TDLU involution, indicated by higher numbers of TDLUs and acini per TDLU, has been associated with higher breast cancer risk among women with benign breast disease (BBD) [16–19]. Further, several breast cancer risk factors, including hormonally related factors such as younger age at menarche and fewer years since menopause, have been shown to be associated with higher TDLU count among women without BBD [20], further supporting the evaluation of TDLUs and their epithelial cell content as an intermediate endpoint for breast cancer. However, little is known about whether circulating EMs, with their proliferative potential, increase breast cancer risk through their associations with higher numbers of TDLUs and acini/TDLU in the breast tissue.
To the best of our knowledge, no study to date has examined the relationships between specific EMs and TDLU involution. One previous study of TDLU involution among women without BBD measured serum estradiol using an immunoassay and reported that elevated estradiol levels were associated with higher TDLU count [21]. Herein, we used a high-performance liquid chromatography-tandem mass spectrometry (LC/MS-MS) assay to refine measurements and extended the analysis to examine detailed patterns of estrogen metabolism in relation to highly reliable measures of TDLUs and acini count/TDLU in the background normal breast tissue from women undergoing diagnostic breast biopsy. We also examined whether the associations for specific EMs were independent of unconjugated estradiol, the bioactive form of estrogen strongly associated with breast cancer risk.
Materials and Methods
Study Population
The National Cancer Institute (NCI) Breast Radiology Evaluation and Study of Tissues (BREAST) Stamp Project is a cross-sectional molecular epidemiologic study of mammographic density conducted among 465 women, aged 40 to 65 years, who were referred for diagnostic image-guided breast biopsy from 2007 to 2010 at the University of Vermont Medical Center. Details of this study have been previously described [22]. Participants had no prior history of breast cancer or cancer treatments, had not undergone breast surgery within 1 year of enrollment, did not have breast implants, and were not taking breast cancer chemoprevention. A standard self-administered questionnaire and a supplementary telephone interview collected information on the participants’ medical history and breast cancer risk factors. Height and weight were measured on the day of the breast biopsy. Blood samples were voluntarily provided by 324 women (70 %) as part of the project. Participant characteristics were similar among women who provided blood and those who did not. A woman was considered postmenopausal if menstrual periods had stopped more than 12 months prior to the interview, she had undergone bilateral oophorectomy, or she had undergone a hysterectomy and was 55 years or older. Among premenopausal women, menstrual cycle length and phase were estimated using the date of their last menstrual period reported at the time of blood collection and the date of the first day of their next menstrual period reported via a postcard returned after the blood collection. Menstrual cycle length was determined by computing the difference in days between the self-reported date of last menstrual period at the time of blood collection and the first day of the next menstrual period following blood collection. Menstrual cycle phase was categorized as luteal if blood was collected within the last 11 days of the menstrual cycle and periovulatory if blood was collected 12–16 days before the end of the menstrual cycle; otherwise, the participants were considered to be in follicular phase. If menstrual cycle length could not be determined due to either a missing or invalid date of the last or next menstrual period, we assumed a 28-day cycle length and counted either forward (if date of last menstrual period was available) or backward (if date of next menstrual period was available) and classified as follows: follicular (blood collected on days 1–10 of the menstrual cycle), periovulatory (days 11–16), and luteal (days 17–28). Mammographic density was measured on the mammogram taken closest in time prior to the breast biopsy date. Volume measures of mammographic density were assessed using single X-ray absorptiometry (SXA) [23]. Final pathologic diagnoses were obtained from pathology reports.
Of the 324 women who provided at least one vial of serum, the current analysis excluded 29 current exogenous hormone users, 19 women with unknown menopausal status, 57 premenopausal women with indeterminate menstrual cycle phase, and 15 women with ≤3.2 mL of serum available. We further excluded 13 perimenopausal women (who had menstrual periods in the last 12 months but had a serum follicle-stimulating hormone (FSH) level of >33.4 IU/L or whose menstrual periods stopped more than 12 months prior to interview but had a FSH level of <23 IU/L and an estradiol level of >37 pg/mL) and five women without biopsy tissue available for research. A total of 186 women (94 premenopausal, 92 postmenopausal) were included in the final analytic population.
Participants provided written informed consent, and the study was approved by Institutional Review Boards at the University of Vermont and the NCI.
Blood Collection and Laboratory Assays
Blood collection [24] and EM hormone assay [25] methods have been described previously. Aliquoted serum vials were stored in liquid nitrogen until their transfer to the Laboratory of Proteomics and Analytical Technologies, Cancer Research Technology Program, Leidos Biomedical Research, Inc. (Frederick, MD) for testing.
Combined concentrations of conjugated and unconjugated forms of each of the 15 individual EMs (estrone, estradiol, 2-hydroxyestrone, 2-hydroxyestradiol, 2-methoxyestrone, 2-methoxyestradiol, 2-hydroxyestrone-3-methyl ether, 4-hydroxyestrone, 4-methoxyestrone, 4-methoxyestradiol, 16a-hydroxyestrone, estriol, 17-epiestriol, 16-ketoestradiol, and 16-epiestriol) and unconjugated concentrations of five EMs (estrogen, estradiol, estriol, 2-methoyxestrone, and 2-methoxyestradiol) in the serum were measured in picomoles per liter (pmol/L) using stable isotope dilution LC-MS/MS [25]. For those five EMs with both combined and unconjugated measurements, their conjugated concentration was estimated by subtracting the unconjugated concentration from the combined concentration. EMs were also grouped by pathways (e.g., parent estrogens, 2-, 4-, and 16-hydroxylation pathways) and pathway ratios (e.g., catechols/methylated catechols). “Sum EMs” was also calculated by adding up all 15 individual EMs. Assay reliability was monitored using 10 % masked quality control samples. Coefficients of variation were <3 %; intraclass correlation coefficients were >99 % for each EM [24].
Morphometric TDLU Assessment
TDLU assessment was performed as described previously [20, 26]. Briefly, hematoxylin and eosin (H&E)-stained sections were digitized at ×20 magnification (Aperio ScanScope CS, Vista, CA) and evaluated using a web-based system (Digital Image Hub software; SlidePath/Leica, Dublin, Ireland). A pathologist (MES) evaluated the images to enumerate normal TDLUs per section and estimated the percent nonfat tissue area in categories (0, 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 95, 99, and 100 %). The lasso tool in Digital Image Hub was used to manually outline and measure total tissue area (square millimeters) per section. Using this information, we computed the number of TDLUs per square millimeter of nonfat tissue area (“TDLU count”). Among women with observable TDLUs, a semi-automated image analysis tool was used to quantify the number of acini per TDLU (“acini count/TDLU”) as previously described [21, 27]. For acini count/TDLU, up to ten TDLUs were reviewed to provide reliable estimates [28] and median values were selected as a single summary measure for each woman. Among women who had mammographic density measured (92 premenopausal, 88 postmenopausal women), we also estimated their total TDLU content in the entire breast (“total TDLU volume”) by multiplying the TDLU count per square millimeter of nonfat tissue area by the summed absolute dense volume of both breasts. A previous study [20] demonstrated high intra-observer agreement (Spearman r > 0.90) with the study pathologist (MES) for the TDLU measures and found moderate inverse correlations between these TDLU measures and the qualitative and subjective impression of TDLU involution, which had been previously linked to mammographic density and breast cancer risk [29].
Statistical Analysis
All analyses were stratified by menopausal status, as levels of serum EM [24, 30] and TDLU measures [20, 26] substantially vary by this characteristic. Each EM measure was categorized into tertiles (T1, T2, and T3 indicate the first, second, and third tertiles) within pre- and postmenopausal women. Zero-inflated Poisson regression (ZIP) [31] models, with a sandwich robust variance estimator [32, 33], were fit to accommodate the count data with excess zeros (zero TDLU count) and to estimate relative risks (RRs) and 95 % confidence intervals (CIs) for the relationship between serum EM levels and TDLU count. In the ZIP models, we standardized the TDLU count by including the nonfat tissue area on the H&E slides as an offset. Among women with at least one observable TDLU, ordinal logistic regression models were used to estimate odds ratios (ORs) and 95 % CIs for the associations between EM levels and median acini count/TDLU, categorized in tertiles. We adjusted all multivariable models for age and other potential confounders. For each outcome, potential confounders (percentage of fat on the H&E slide, body mass index (kg/m2), smoking status, age at menarche, first-degree family history of breast cancer, age at first birth/parity, and biopsy type) were included in the multivariable models only if they were associated with both the exposure and the outcome, assessed separately in pre- and postmenopausal women. Since additional adjustment for menstrual phase in premenopausal women and age at menopause in postmenopausal women did not change the results, these variables were not included in the final models. In a separate model, we additionally adjusted for unconjugated estradiol to examine the association of each EM independent of unconjugated estradiol. Tests for trend were performed by including exposures in the model as a continuous variable (EM tertiles as an ordinal trend).
In the secondary analyses, we stratified premenopausal women by their menstrual phase (follicular, periovulatory, and luteal) because premenopausal hormone levels vary by menstrual cycle phase. In addition, postmenopausal women were stratified by the median percentage of fat on the H&E slides (≥40% vs. <40 %) to assess whether associations differ among women who tend to have a higher proportion of breast adipose tissue which may serve as a reservoir of hormones and cytokines [34]. All stratified analyses were adjusted only for age due to small sample sizes within subgroups. In an additional secondary analysis, we also estimated ORs and 95 % CIs for the associations between EM levels and total TDLU volume (in quintile categories) using ordinal logistic regression models, adjusting for potential confounders. In the sensitivity analyses, we repeated analyses after excluding women who may have had extreme values of EMs and TDLU measures: women who were diagnosed with breast carcinoma (in situ or invasive) at biopsy (n = 9 premenopausal, n = 22 postmenopausal women), current smokers (n = 7 premenopausal, n = 11 postmenopausal women), and women who had used hormones within the prior year (n = 6 premenopausal, n = 10 postmenopausal women).
All statistical tests were two-sided with 5 % type I error. Given the correlated exposures (r = 0.19–0.97 among the 15 EMs) (Supplementary Tables 1 and 2), we also applied a false discovery rate (FDR) adjustment for multiple testing (44 tests per outcome) in the secondary analyses; however, none of the p values for trend, with the exception of few EMs in the stratified analysis, remained statistically significant after the adjustment (adjusted p > 0.05). The ZIP models with the robust variance were estimated using R software, version 3.2.4, and all other analyses were conducted with SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
Results
Study Population Characteristics
The mean age at biopsy was 45.8 years for premenopausal women (n = 94) and 57.2 years for postmenopausal women (n = 92) (Table 1). Most women were non-Hispanic white (92 %), parous (76 %), and had used oral contraceptives in the past (85 %). Compared with premenopausal women, postmenopausal women, on average, had lower dense breast volume. In addition, postmenopausal women were more likely to have had menarche at age ≤12 years, to be ever smokers, and to have been diagnosed with in situ or invasive carcinoma at breast biopsy. As expected, median TDLU count and median acini count/TDLU were higher in premenopausal women than in postmenopausal women (among women with at least one observable TDLU:41.9 vs. 22.9 TDLUs per 100 mm2 nonfat tissue area, and 15.8 vs. 8.0 acini/TDLU). Median serum EM levels for pre- and postmenopausal women are presented in Supplementary Table 3. Among premenopausal women, median serum EM levels were generally lowest in the follicular phase and highest in the periovulatory phase.
Premenopausal Women
Among premenopausal women, most individual EMs were generally positively associated with TDLU count (Table 2). Specifically, higher levels of unconjugated estradiol (RRT3 vs. T1 = 1.74, 95 % CI = 1.06–2.87, p trend = 0.03), 2-hydroxyestrone (RRT3 vs. T1 = 1.74, 95 % CI = 1.01–3.01, p trend = 0.04), and 4-hydroxyestrone (RRT3 vs. T1 = 1.74, 95 % CI = 0.99–3.06, p trend = 0.04) were statistically significantly associated with higher TDLU count. The associations for 2-hydroxyestrone and 4-hydroxyestrone remained statistically significant after additional adjustment for unconjugated estradiol (p trend = 0.03). The ratio of 4-pathway to 16-pathway EMs (RRT3 vs. T1 = 1.92, 95 % CI = 1.22–3.04, p trend = 0.01) was also significantly associated with higher TDLU count independent of unconjugated estradiol.
Although we had limited sample sizes within the strata defined by menstrual cycle phase, we observed suggestive heterogeneity in associations by menstrual phase for some EMs (Supplementary Table 4). Most EMs measured in the follicular phase were positively associated with TDLU count, consistent with findings for all premenopausal women combined. However, associations for luteal phase EMs were variable, showing positive associations for levels of 2- and 4-pathway catechols only. Periovulatory levels of estriol were inversely associated with TDLU count.
Among premenopausal women with observable TDLUs, higher levels of 2-hydroxyestradiol, conjugated estriol, and 16-epiestriol were associated with lower acini count/TDLU (Supplementary Table 5).
Postmenopausal Women
Among postmenopausal women, higher levels of estradiol (RRT3 vs. T1 = 2.09, 95 % CI = 1.01–4.30, p trend = 0.04) and 16α-hydroxyestrone (RRT3 vs. T1 = 2.27, 95 % CI = 1.29–3.99, p trend = 0.02) were significantly associated with higher TDLU count (Table 3). After additional adjustment for unconjugated estradiol, these associations remained statistically significant, and higher levels of estrone were also significantly associated with higher TDLU count (p trend = 0.04).
When we stratified analyses by percent fat on the H&E slides, we observed some heterogeneity in associations (Supplementary Table 6). The associations between EMs and TDLU count were generally positive among women with <40 % fat on the slides. Among the women with ≥40 % fat on the slides, the associations were inverse for most 2- and 4-pathway EMs and 17-epiestriol; however, sample sizes within the strata were small.
Among postmenopausal women with observable TDLUs, EMs were generally associated with higher acini count/TDLU; the associations were statistically significant for conjugated estrone and 2-and 4-pathway catechols (all p trend < 0.05) (Supplementary Table 5).
Sensitivity Analyses Among Pre- and Postmenopausal Women
When we accounted for total mammographic dense volume in order to estimate the total TDLU volume in the entire breast, individual EMs were generally positively associated with the total TDLU volume in both pre- and postmenopausal women, but the confidence intervals were wider than those observed for the relationships between EMs and TDLU count (Supplementary Table 7).
Results were similar when excluding pre- and postmenopausal women who were diagnosed with in situ or invasive carcinoma at biopsy, excluding current smokers or excluding women who had used exogenous hormones within the prior year (data not shown).
Discussion
In this cross-sectional study of women undergoing diagnostic image-guided breast biopsy, higher levels of serum estradiol were associated with higher TDLU count in both pre- and postmenopausal women. Independent of unconjugated estradiol, levels of 2- and 4-pathway catechols in premenopausal women and levels of 16α-hydroxyestrone in postmenopausal women were also associated with higher TDLU count. Among postmenopausal women, higher levels of parent estrogens and 2- and 4-pathway catechols were associated with higher acini count/TDLU. Our findings suggest opportunities for future investigations evaluating whether EMs increase breast cancer risk through maintaining higher numbers of TDLUs and acini/TDLU in the breast tissue.
To our knowledge, this is the first study to evaluate a panel of serum EMs in relation to TDLU measures. Among women without BBD, we previously reported that higher levels of serum estradiol were associated with higher TDLU count in postmenopausal women and premenopausal women in the follicular phase [21]. In the present study of women referred to diagnostic breast biopsy, findings were consistent; furthermore, we demonstrated associations of specific EMs with TDLU count independent of unconjugated estradiol, the bioactive form of estrogen strongly associated with breast cancer risk.
Prior studies of postmenopausal women have consistently found that higher circulating levels of parent estrogens are associated with higher breast cancer risk, whereas a higher ratio of 2-pathway EMs to parent estrogens is associated with lower risk [3–5]. A potential underlying mechanism for these associations is that when EMs bind ER, they facilitate cell proliferation and reduce apoptosis [9], thereby maintaining high numbers of TDLUs in the breasts and elevating breast cancer risk. Compared with 4- or 16-pathway EMs, 2-pathway EMs may dissociate faster from ER [35] and may be more rapidly cleared from the body than parent estrogens [10, 11], therefore producing less proliferative stimulus of breast epithelial cells. In the present study, although statistically nonsignificant, we found a similar pattern of associations between postmenopausal EMs and TDLU count, lending support to the notion that TDLU involution may mediate the effects of EMs on postmenopausal breast cancer risk.
In premenopausal women, we observed suggestive heterogeneity in associations between EMs and TDLU count by menstrual cycle phase, although interpretation was limited by small numbers. As hormone levels fluctuate across the menstrual cycle and the interaction of EMs with other hormones (e.g., progesterone) at different times of the menstrual cycle may influence the associations, longitudinal studies are needed to confirm the differing effects of follicular vs. luteal hormone exposure on breast cancer risk and its intermediate endpoints.
Our findings relating serum EMs to TDLU count are consistent with previous studies of EMs and mammographic density, which, like TDLU count, is also thought to be an intermediate marker of breast cancer risk [36]. Luteal levels of serum estriol and postmenopausal levels of serum parent estrogens, 2-, 4-, and 16-pathway EM groups have previously been shown to be weakly but positively associated with percent and absolute measures of dense area in the same study population [24]. In the studies of urinary EMs and mammographic density [37, 38], 2-pathway catechols in premenopausal women were suggestively positively associated with higher percent mammographic density [37] and the ratios of 2-, 4-, and 16-pathway EMs to parent estrogens in postmenopausal women were inversely associated with percent and absolute dense area [38]. Previous data suggest that TDLU involution is inversely related to mammographic density [26, 39], suggesting that further etiologic research incorporating hormone measures with these features of breast composition are warranted.
Although measures of TDLU involution and mammographic density are closely related [26, 39], studies suggest that they are independently associated with breast cancer risk [29]. TDLUs are the major epithelial structures of the breast where breast cancers arise [14], whereas mammographic density reflects stromal and epithelial tissue [40, 41]. In the present study, we observed significant associations with TDLU count, suggesting that EMs may have a direct influence on epithelial tissue and specifically TDLU content.
When evaluating EM associations with the total TDLU volume, we generally observed positive associations among both pre- and postmenopausal women. In this analysis, using a combinatorial metric that incorporated both TDLU number on a biopsy tissue section and absolute dense volume from mammography in the outcome calculation contributed to larger variances, resulting in wide confidence intervals. Future efforts to more directly study the total TDLU count or total epithelial content in the entire breast may give more insight into the overall process of TDLU involution and its relationship with breast cancer risk.
As acini count/TDLU is weakly correlated with TDLU count [20, 26], TDLU number and acinar content may indicate different biological processes or stages of TDLU involution. TDLU involution may occur in women by either reducing the number of TDLUs or reducing the number of acini within the TDLUs, both resulting in decreased overall epithelial content in the breast. Finally, because women must have had at least one observable TDLU to be included in the analysis for acini count/TDLU, we may have had limited statistical power to detect some modest associations between serum EMs and acini count/TDLU.
We acknowledge several limitations of this study. Because we performed multiple tests with 44 different individual EMs, pathway groups, and ratios, some of the significant findings we observed could be due to chance. The majority of our findings did not remain statistically significant after the adjustment for multiple testing, potentially due to limited sample size and the possible modest effects of EMs on TDLU involution. However, given the exploratory nature of the study, this study serves as a hypothesis-generating analysis that may provide a basis for future studies. In the stratified analyses, we had a limited sample size in each subgroup, and thus we were not able to adjust for potential confounders in these models other than age. However, in our overall analyses, the age-adjusted and the multivariable-adjusted results were very similar.
Despite the limitations, this study has important strengths. We used reproducible, standardized measures of TDLU involution. Use of the LC/MS-MS assay also allowed us to comprehensively measure 15 individual EMs with high sensitivity, specificity, and reliability [25, 30], even for the lower levels of estradiol characteristic of postmenopausal women [42–44]. In summary, we observed that serum levels of estradiol and certain individual EMs were generally associated with higher TDLU count among both pre- and postmenopausal women with BBD. These findings were independent of circulating unconjugated estradiol, suggesting a possible role of EMs in influencing TDLU involution. As both estrogen metabolism and TDLU involution are potentially modifiable breast cancer risk factors, this study provides impetus for further research incorporating these metrics as a means of refining risk assessment for breast cancer.
References
Key T, Appleby P, Barnes I, Reeves G, Endogenous Hormones Breast Cancer Collaborative Group (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94(8):606–616
Endogenous Hormones and Breast Cancer Collaborative Group, Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoff man-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P (2013) Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 14(10):1009–1019. doi:10.1016/S1470-2045(13)70301-2
Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, Buys SS, Isaacs C, Keefer LK, Veenstra TD, Berg CD, Hoover RN, Ziegler RG (2012) Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 104(4):326–339. doi:10.1093/jnci/djr531
Dallal CM, Tice JA, Buist DS, Bauer DC, Lacey JV Jr, Cauley JA, Hue TF, Lacroix A, Falk RT, Pfeiffer RM, Fuhrman BJ, Veenstra TD, Xu X, Brinton LA (2014) Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B∼FIT. Carcinogenesis 35(2):346–355. doi:10.1093/carcin/bgt367
Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, Gierach GL (2013) Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res BCR 15(2):R34. doi:10.1186/bcr3416
Santen RJ, Yue W, Wang JP (2015) Estrogen metabolites and breast cancer. Steroids 99(Pt A):61–66. doi:10.1016/j.steroids.2014.08.003
Santen R, Cavalieri E, Rogan E, Russo J, Guttenplan J, Ingle J, Yue W (2009) Estrogen mediation of breast tumor formation involves estrogen receptor-dependent, as well as independent, genotoxic effects. Ann N Y Acad Sci 1155:132–140. doi:10.1111/j.1749-6632.2008.03685.x
Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH (2003) Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J Steroid Biochem Mol Biol 87(1):1–25
Seeger H, Wallwiener D, Kraemer E, Mueck AO (2006) Comparison of possible carcinogenic estradiol metabolites: effects on proliferation, apoptosis and metastasis of human breast cancer cells. Maturitas 54(1):72–77. doi:10.1016/j.maturitas.2005.08.010
Kono S, Merriam GR, Brandon DD, Loriaux DL, Lipsett MB, Fujino T (1983) Radioimmunoassay and metabolic clearance rate of catecholestrogens, 2-hydroxyestrone and 2-hydroxyestradiol in man. J Steroid Biochem 19(1B):627–633
Pfeiffer E, Graf E, Gerstner S, Metzler M (2006) Stimulation of estradiol glucuronidation: a protective mechanism against estradiol-mediated carcinogenesis? Mol Nutr Food Res 50(4–5):385–389. doi:10.1002/mnfr.200500198
Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D (2000) Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J Natl Cancer Inst Monogr 27:75–93
Yager JD (2000) Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr 27:67–73
Wellings SR, Jensen HM, Marcum RG (1975) An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst 55(2):231–273
Hutson SW, Cowen PN, Bird CC (1985) Morphometric studies of age related changes in normal human breast and their significance for evolution of mammary cancer. J Clin Pathol 38(3):281–287
Baer HJ, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Tamimi RM (2009) Lobule type and subsequent breast cancer risk: results from the Nurses’ Health Studies. Cancer 115(7):1404–1411. doi:10.1002/cncr.24167
Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, Thompson RA, Melton LJ 3rd, Goode EL, Visscher DW (2006) Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst 98(22):1600–1607. doi:10.1093/jnci/djj439
McKian KP, Reynolds CA, Visscher DW, Nassar A, Radisky DC, Vierkant RA, Degnim AC, Boughey JC, Ghosh K, Anderson SS, Minot D, Caudill JL, Vachon CM, Frost MH, Pankratz VS, Hartmann LC (2009) Novel breast tissue feature strongly associated with risk of breast cancer. J Clin Oncol 27(35):5893–5898. doi:10.1200/JCO.2008.21.5079
Figueroa J, Pfeiffer RM, Brinton L, Palakal MM, Degnim AC, Radisky DC, Hartmann LC, Frost C, Stallings Mann ML, Papathomas D, Hewitt S, Visscher D, Sherman M (2015) Standardized measures of lobular involution and subsequent breast cancer risk among women with benign breast disease. Cancer Res. doi:10.1158/1538-7445.AM2015-4682.
Figueroa JD, Pfeiffer RM, Patel DA, Linville L, Brinton LA, Gierach GL, Yang XR, Papathomas D, Visscher D, Mies C, Degnim AC, Anderson WF, Hewitt S, Khodr ZG, Clare SE, Storniolo AM, Sherman ME (2014) Terminal duct lobular unit involution of the normal breast: implications for breast cancer etiology. J Natl Cancer Inst. doi:10.1093/jnci/dju286
Khodr ZG, Sherman ME, Pfeiffer RM, Gierach GL, Brinton LA, Falk RT, Patel DA, Linville LM, Papathomas D, Clare SE, Visscher DW, Mies C, Hewitt SM, Storniolo AM, Rosebrock A, Caban JJ, Figueroa JD (2014) Circulating sex hormones and terminal duct lobular unit involution of the normal breast. Cancer Epidemiol Biomarkers Prev 23(12):2765–2773. doi:10.1158/1055-9965.EPI-14-0667
Gierach GL, Geller BM, Shepherd JA, Patel DA, Vacek PM, Weaver DL, Chicoine RE, Pfeiffer RM, Fan B, Mahmoudzadeh AP, Wang J, Johnson JM, Herschorn SD, Brinton LA, Sherman ME (2014) Comparison of mammographic density assessed as volumes and areas among women undergoing diagnostic image-guided breast biopsy. Cancer Epidemiol Biomarkers Prev 23(11):2338–2348. doi:10.1158/1055-9965.EPI-14-0257
Malkov S, Wang J, Kerlikowske K, Cummings SR, Shepherd JA (2009) Single x-ray absorptiometry method for the quantitative mammographic measure of fibroglandular tissue volume. Med Phys 36(12):5525–5536
Gierach GL, Patel DA, Falk RT, Pfeiffer RM, Geller BM, Vacek PM, Weaver DL, Chicoine RE, Shepherd JA, Mahmoudzadeh AP, Wang J, Fan B, Herschorn SD, Xu X, Veenstra T, Fuhrman B, Sherman ME, Brinton LA (2015) Relationship of serum estrogens and metabolites with area and volume mammographic densities. Horm Cancer 6(2–3):107–119. doi:10.1007/s12672-015-0216-3
Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG (2007) Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem 79(20):7813–7821. doi:10.1021/ac070494j
Gierach GL, Patel DA, Pfeiffer RM, Figueroa JD, Linville L, Papathomas D, Johnson JM, Chicoine RE, Herschorn SD, Shepherd JA, Wang J, Malkov S, Vacek PM, Weaver DL, Fan B, Mahmoudzadeh AP, Palakal M, Xiang J, Oh H, Horne HN, Sprague BL, Hewitt SM, Brinton LA, Sherman ME (2016) Relationship of terminal duct lobular unit involution of the breast with area and volume mammographic densities. Cancer Prev Res 9(2):149–158. doi:10.1158/1940-6207.CAPR-15-0282
Rosebrock A, Caban JJ, Figueroa J, Gierach G, Linville L, Hewitt S, Sherman M (2013) Quantitative analysis of TDLUs using adaptive morphological shape techniques. Proc SPIE Int Soc Opt Eng 8676. doi:10.1117/12.2006619
Yang XR, Figueroa JD, Falk RT, Zhang H, Pfeiffer RM, Hewitt SM, Lissowska J, Peplonska B, Brinton L, Garcia-Closas M, Sherman ME (2012) Analysis of terminal duct lobular unit involution in luminal A and basal breast cancers. Breast Cancer Res BCR 14(2):R64. doi:10.1186/bcr3170
Ghosh K, Vachon CM, Pankratz VS, Vierkant RA, Anderson SS, Brandt KR, Visscher DW, Reynolds C, Frost MH, Hartmann LC (2010) Independent association of lobular involution and mammographic breast density with breast cancer risk. J Natl Cancer Inst 102(22):1716–1723. doi:10.1093/jnci/djq414
Fuhrman BJ, Xu X, Falk RT, Dallal CM, Veenstra TD, Keefer LK, Graubard BI, Brinton LA, Ziegler RG, Gierach GL (2014) Assay reproducibility and interindividual variation for 15 serum estrogens and estrogen metabolites measured by liquid chromatography-tandem mass spectrometry. Cancer Epidemiol Biomarkers Prev 23(12):2649–2657. doi:10.1158/1055-9965.EPI-14-0438
Long JS (1997) Count outcomes: regression models for counts, 1st edn. SAGE Publications, Inc., Thousand Oaks
Zeileis A (2004) Econometric computing with HC and HAC covariance matrix estimators. J Stat Softw 11(10):1–17
Zeileis A (2006) Object-oriented computation of sandwich estimators. J Stat Softw 16(9):1–16
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6):2548–2556. doi:10.1210/jc.2004-0395
Barnea ER, MacLusky NJ, Naftolin F (1983) Kinetics of catechol estrogen-estrogen receptor dissociation: a possible factor underlying differences in catechol estrogen biological activity. Steroids 41(5):643–656
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD (2005) Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 6(10):798–808. doi:10.1016/S1470-2045(05)70390-9
Bertrand KA, Eliassen AH, Hankinson SE, Gierach GL, Xu X, Rosner B, Ziegler RG, Tamimi RM (2012) Urinary estrogens and estrogen metabolites and mammographic density in premenopausal women. Breast Cancer Res Treat 136(1):277–287. doi:10.1007/s10549-012-2240-0
Fuhrman BJ, Brinton LA, Pfeiffer RM, Xu X, Veenstra TD, Teter BE, Byrne C, Dallal CM, Barba M, Muti PC, Gierach GL (2012) Estrogen metabolism and mammographic density in postmenopausal women: a cross-sectional study. Cancer Epidemiol Biomarkers Prev 21(9):1582–1591. doi:10.1158/1055-9965.EPI-12-0247
Ghosh K, Hartmann LC, Reynolds C, Visscher DW, Brandt KR, Vierkant RA, Scott CG, Radisky DC, Sellers TA, Pankratz VS, Vachon CM (2010) Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol 28(13):2207–2212. doi:10.1200/JCO.2009.23.4120
Boyd NF, Lockwood GA, Martin LJ, Knight JA, Byng JW, Yaffe MJ, Tritchler DL (1998) Mammographic densities and breast cancer risk. Breast Dis 10(3–4):113–126
Guo YP, Martin LJ, Hanna W, Banerjee D, Miller N, Fishell E, Khokha R, Boyd NF (2001) Growth factors and stromal matrix proteins associated with mammographic densities. Cancer Epidemiol Biomarkers Prev 10(3):243–248
Dorgan JF, Fears TR, McMahon RP, Aronson Friedman L, Patterson BH, Greenhut SF (2002) Measurement of steroid sex hormones in serum: a comparison of radioimmunoassay and mass spectrometry. Steroids 67(3–4):151–158
McShane LM, Dorgan JF, Greenhut S, Damato JJ (1996) Reliability and validity of serum sex hormone measurements. Cancer Epidemiol Biomarkers Prev 5(11):923–928
Rinaldi S, Dechaud H, Biessy C, Morin-Raverot V, Toniolo P, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Secreto G, Ciampi A, Riboli E, Kaaks R (2001) Reliability and validity of commercially available, direct radioimmunoassays for measurement of blood androgens and estrogens in postmenopausal women. Cancer Epidemiol Biomarkers Prev 10(7):757–765
Acknowledgments
The authors are indebted to the participants in the BREAST Stamp Project for their outstanding cooperation and to the physicians, pathologists, nurses, technologists, and interviewers for their efforts in the field. The authors thank Clair Bove, Patricia Lutton, Ellen Young, and Aileen Burke for research assistance. We also thank Janet Lawler-Heaver and Kerry Grace Morrissey from Westat for study management support, Patricia Madigan from NCI for editorial assistance, and Jane Demuth at Information Management Services and Clara Bodelon from NCI for data support and analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Participants provided written informed consent, and the study was approved by Institutional Review Boards at the University of Vermont and the NCI.
Financial Support
This study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute. Breast Cancer Research Stamp Funds (MES, LAB) and cooperative agreement U01CA70013 (BMG, PMV, DLW, REC) from the National Cancer Institute funded some of the data collection for this study. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Conflict of Interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 70 kb)
Supplementary Table 2
(DOC 70 kb)
Supplementary Table 3
(DOC 70 kb)
Supplementary Table 4
(DOC 123 kb)
Supplementary Table 5
(DOC 111 kb)
Supplementary Table 6
(DOC 120 kb)
Supplementary Table 7
(DOC 110 kb)
Rights and permissions
About this article
Cite this article
Oh, H., Khodr, Z.G., Sherman, M.E. et al. Relation of Serum Estrogen Metabolites with Terminal Duct Lobular Unit Involution Among Women Undergoing Diagnostic Image-Guided Breast Biopsy. HORM CANC 7, 305–315 (2016). https://doi.org/10.1007/s12672-016-0265-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-016-0265-2