Abstract
Tumor cell proliferation and progression of breast cancer are influenced by female sex steroids. However, not all breast cancer patients respond to aromatase inhibitors (AI), and many patients become unresponsive or relapse. Recent studies demonstrate that not only estrogens but also androgens may serve as regulators of estrogen-responsive as well as estrogen-unresponsive human breast cancers. However, the mechanism underlying these androgenic actions has remained relatively unknown. Therefore, in this study, we evaluated the effects of AI upon the expression of enzymes involved in intratumoral androgen production including 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5), 5α-reductase types 1 and 2 (5αRed1 and 5αRed2) as well as androgen receptor (AR) levels and correlated the findings with therapeutic responses including Ki67 labeling index (Ki67). Eighty-two postmenopausal invasive ductal carcinoma patients were enrolled in CAAN study from November 2001 to April 2004. Pre- and post-treatment specimens of 29 cases were available for this study. The status of 17βHSD5, 5αRed1, 5αRed2, and Ki67 in pre- and post-treatment specimens were evaluated. The significant increments of 5αRed2 as well as AR were detected in biological response group whose Ki67 LI decreased by more than 40% of the pre-treatment level. This is the first study demonstrating an increment of 5αRed2 and AR in the group of the patients associated with Ki67 decrement following AI treatment. These results suggest that increased 5αRed2 and AR following AI treatment may partly contribute to reduce the tumor cell proliferation through increasing intratumoral androgen concentrations and its receptor.
Similar content being viewed by others
Introduction
Breast cancer is the most common malignancy among women worldwide and the leading cause of cancer-related death in many countries [1, 2]. Hormones, especially sex steroid hormones, play a pivotal role in endocrine-mediated tumorigenesis and have been demonstrated to influence carcinoma cell growth and progression [3, 4]. Among these sex steroids, estrogens, especially estradiol or E2, a biologically potent estrogen, has been demonstrated to play pivotal roles in cell proliferation, development, and invasion of these hormone-dependent breast carcinoma cells [4, 5]. Aromatase inhibitors (AI) have been demonstrated to be more effective and to have fewer side effects in estrogen receptor (ER)-positive breast cancer patients than the conventional anti-estrogen tamoxifen [6–8]. However, some patients did not respond to this therapy or developed clinical resistance during the course of this therapy [9]. Therefore, it becomes very important to evaluate the mechanisms of these clinical resistances to AI therapy in estrogen receptor positive breast cancer patients. Results of several previous studies demonstrated that androgens exert opposing effects upon the growth and development as well as upon an inhibition of the proliferation of breast carcinoma cells [10, 11], although some controversies existed [12]. In addition, estrogens and androgens have been both reported to be locally produced in breast carcinoma tissue in an intracrine manner [13, 14]. Androgen receptor (AR) is commonly expressed in human breast carcinoma tissues [15]. These data of in situ production of androgen and the presence of AR in breast carcinoma suggest potentially important roles of androgens in breast carcinomas. In particular, androgen producing enzymes, such as 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5; conversion from circulating androstenedione to testosterone) and 5α-reductase types 1 and 2 (5αRed1 and 5αRed2, respectively; reduction of testosterone to 5α-dihydrotestosterone (DHT)) have been reported to be abundantly expressed in breast carcinoma tissues [16]. Especially, in situ production of DHT has been reported in breast cancer tissues [17]. This locally produced DHT then binds with the highest affinity to AR and promotes AR transcriptional activity [16].
We have previously demonstrated an association between the status of intratumoral androgenic enzymes, 5αRed1, and DHT concentration in the breast carcinoma tissue and an inverse correlation between intratumoral DHT concentration and aromatase expression in cell culture experiments [17]. Results of our previous study above indicated that aromatase, whose substrates include testosterone, may act as a negative regulator for in situ production of DHT in breast carcinoma tissue. Therefore, the alterations of these in situ androgen metabolisms following AI treatment can provide very important information toward a better understanding of the changes of local endocrine environment associated with estrogen depletion. Especially, the comparison of the specimens between pre- and post-AI treatment in neoadjuvant therapy may provide important information as to the changes of intratumoral intracrine environment caused by AI. We have recently reported significant increment of the enzymes; estrogen sulfatase and 17β-hydroxysteroid dehydrogenase type 1, the enzymes also involved in intratumoral estrogen production, following AI therapy, which may represent the compensatory response of breast carcinoma tissues to estrogen deprivation state [18]. In addition, Takagi et al. has also recently demonstrated the increment of intratumoral DHT concentration and17β-hydroxysteroid dehydrogenase type 2 (17βHSD2) expressions in breast carcinoma tissues following exemestane treatment and further reported that 17βHSD2 expression was induced by both DHT and exemestane in a dose dependent fashion in their in vitro studies [19]. However, to the best of our knowledge, the alterations of major androgen producing enzymes such as 17βHSD5, 5αRed1, and 5αRed2 before and after AI treatment of breast cancer patients have not been reported at all (Figs. 1 and 2).
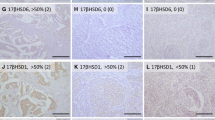
Representative illustrations of immunohistochemistry: 17βHSD5 (a), 5αRed1 (b), 5αRed2 (c), and AR (d) in one case of invasive ductal carcinoma. Immunoreactivity of 17βHSD5, 5αRed1, and 5αRed2 were detected in the cytoplasm of invasive ductal carcinoma cells while those of AR in the nucleus. Original magnification, ×200
Therefore, in this study, we evaluated the alterations of these enzymes including 17βHSD5, 5αRed1 and 5αRed2, and AR expression in breast carcinoma tissue before and after the neoadjuvant AI treatment using the immunohistochemistry (IHC). We then correlated the obtained findings with the alteration of Ki67 of individual patients and the changes of ER, progesterone receptor (PgR), and human epidermal growth factor receptor type 2 (Her2) in breast carcinoma tissues before and after the therapy in order to further understand these changes of intratumoral androgen producing pathways. In particular, we evaluated the clinical and biological significance of intratumoral androgenic enzymes, especially 5αRed2, in association with the decreased Ki67 from estrogen depletion caused by AI therapy.
Materials and Methods
Breast Carcinoma Cases
The specimens available for examinations in this study were pre- and post-treatment samples obtained from Celecoxib Anti-aromatase Neoadjuvant trial (CAAN trial). This was a neoadjuvant clinical trial conducted, from November 2001 to April 2004, at The University of Hong Kong and Queen Mary Hospital, Hong Kong [20]. The study design had been reported previously [20] but, in brief, all 82 patients enrolled in this neoadjuvant study were postmenopausal women with histological proof of invasive ductal breast carcinoma and positive ER/PgR status determined by the IHC analysis [20]. Informed consents had been obtained from all the patients prior to their enrollment into this trial, which had been approved by the local ethics committee. In CAAN trial study, it was conducted to investigate the efficacy and safety of neoadjuvant therapy combining AI with COX-2 inhibitor. According to the protocol of CAAN trial, the patients were randomly assigned to receive exemestane 25 mg daily and celecoxib 400 mg twice daily (group A, n = 30), exemestane 25 mg daily (group B, n = 24) and letrozole 2.5 mg daily (group C, n = 28), respectively. Each patient was treated for 3 months and surgery was performed within 7 days after the treatment. As reported previously, there were no significant differences in term of clinical and pathological responses among these three different treatment groups [20]. Therefore, the responses toward AI therapy were by no means influenced by the concurrent use of celecoxib.
The pre- and post-treatment specimens of 29 patients were available for this pathological response and IHC evaluation study. According to the protocol of CAAN trial, these 29 patients were randomly assigned to receive the treatment as follows (group A, n = 10; group B, n = 8; and group C, n = 11). Their mean age was 74.6 years (range, 51–93 years).
Pathological Response
Tissue sections of the same tumors from pre-treatment core needle biopsies and final surgical specimens were obtained and assessed for the changes in cellularity and degree of fibrosis in hematoxylin–eosin-stained slides. Pathological response was categorized, using the modified criteria described by Miller et al. [21] and assessed as follows: complete when there was no evidence of carcinoma cell at the original tumor site; partial response when histological decrement in cellularity and/or increment in fibrosis was detected; or no change/nonresponse, by two of the authors above (NC and MC).
Immunohistochemistry
All immunohistological investigations were performed on the pre-treatment core needle biopsies and final surgical specimens. One 4-μm section of each submitted paraffin blocks of pre- and post-treatment specimens were stained with hematoxylin–eosin to verify an adequate number of invasive breast carcinoma cells and the quality of fixation in order to determine the suitability of further IHC analysis. In brief, serial tissue sections (4-μm) were prepared from selected blocks and IHC was performed to immunolocalize ER, PgR, Her2, Ki67, AR, 17βHSD5, 5αRed1, and 5αRed2, as described previously [17, 22]. A Histofine Kit (Nichirei, Tokyo, Japan), which employs the streptavidin-biotin amplification method, was used for IHC staining. The lists of primary antibodies used in our present study, the working dilutions of individual antibodies, the details of antigen retrieval methods, the sources of antibodies and the details of positive and negative controls were all summarized in Table 1. The antigen–antibody complex was visualized with 3, 3′-diaminobenzidine (DAB) solution (1 mM DAB, 50 mM Tris–HCl buffer (pH 7.6), and 0.006% H2O2), and counterstained with hematoxylin.
The immunostained slides were independently evaluated by two of the authors (NC and TS), blinded to clinical outcome of individual patients. 17βHSD5, 5αRed1 and 5αRed2 immunoreactivity were evaluated using a semi-quantitative method as follows: score 2, >50% positive cells; score 1, 1–50% positive cells; and score 0, no immunoreactivity, as previously described by Suzuki et al. [23]. Evaluation of Ki67 was performed by counting of 1,000 carcinoma cells or more from each cases and the percentage of immunoreactivity was subsequently determined as a labeling index (LI) [24].
In addition, the Ki67 LI was then subclassified, using the criteria reported by Miller et al. [21], into three different groups according to the percentage of Ki67 alterations after treatment as follows: group1; increased group, the Ki67 LI in this group was associated with an increment after therapy, group2; no change group, the Ki67 LI demonstrated unchanged or reduction for less than 40% of the pre-treatment level, group3; decreased group, the Ki67 LI demonstrated the reduction for more than 40% of the pre-treatment level. ER, PgR, and AR immunoreactivity were scored by assigning proportion and intensity scores, according to Allred’s procedure [25]. In brief, a proportion score represented the estimated proportion of immunopositive tumor cells as follows: 0 (none), 1 (<1/100), 2 (1/100 to 1/10), 3 (1/10–1/3), 4 (1/3 to 2/3), and 5 (>2/3). An intensity score represented the average immunointensity of the positive cells as follows: 0 (none), 1 (weak), 2 (intermediate), and 3 (strong). Any nuclear discernible immunoreactivity in breast carcinoma cells were counted toward both proportion and intensity scores. The proportion and intensity scores were then added to obtain a total score that could range from 0 to 8.The membrane staining pattern was estimated in Her2 IHC and scored on a scale of 0 to 3 [26].
Statistical Analysis
The Kruskal–Wallis test was used to compare the pre-treatment IHC scores of all biological markers according to three groups of AI treatment in individual patients. The Wilcoxon matched-pairs signed ranks test was employed in order to determine the mean differences between pre- and post-treatment IHC scores of individual biological markers in relation to the pathological responses status and the alterations of Ki67 LI. The correlations among intratumoral androgenic enzymes (17βHSD5, 5αRed1, and 5αRed2) before and after AI treatment were analyzed using Spearman’s rank nonparametric correlation test. Logistic regression analysis was conducted to determine whether the changes in androgenic enzymes, especially 5αRed2, predicted for decreased Ki67 LI or response group. The statistically significance was considered the p value < 0.05.
Results
Biopsies from 29 patients who had been treated with exemestane and celecoxib (group A, n = 10), exemestane (group B, n = 8), or letrozole (group C, n = 11), were available for evaluation of pathological response assessment and IHC studies. Pathological responders and nonresponders were 7 (24.1%) and 22 cases (75.9%), respectively.
Immunohistochemistry
The median of pre-treatment individual biological markers were compared but demonstrated no statistical significance (Nonparametric ANOVAs; Data not shown). We then analyzed the changes of IHC scores of all biological markers after the treatment. The statistically significant reduction in PgR expression and Ki67 LI were detected (p = 0.0017 and p = 0.0439, respectively), as previously reported in letrozole [21], anastrozole [27], and exemestane [22] neoadjuvant treatment but the expression levels of both ER and AR were increased (p = 0.015 and p = 0.0127, respectively). In addition, the expressions of intratumoral androgenic enzymes were increased but theses increments did not reach statistical significance (Table 2).
An Association of Alterations of Intratumoral Androgenic Enzymes and Ki67 LI
Differences of the individual enzymes and other biological markers between pre- and post-treatment were evaluated according to those categories of Ki67 LI described above. Immunoreactivity of ER, PgR, Her2, AR, 17βHSD5, 5αRed1, and 5αRed2 in pre-treatment specimens were not significantly different among these three different groups of Ki67 LI changes (nonparametric ANOVAs; data not shown). In group 1 or whose Ki67 LI increased after the therapy and group 2 or whose Ki67 LI unchanged or decreased with less than 40% of the pre-treatment level, no statistically significant difference was detected among any of intratumoral enzymes and biomarkers examined between the specimens before and after the treatment. In group 3 or whose Ki67 LI decreased with more than 40% of the pre-treatment level, the significant increment of 5αRed2 and AR and decrement of PgR expression were demonstrated in this study (p = 0.025, p = 0.039, and p = 0.005, respectively),whereas the expression of other biological markers did not show any statistically significances (Table 3).
Correlation Among Intratumoral Androgenic Enzymes Before and After AI Treatment
We then examined the correlation between IHC scores of intratumoral enzymes in tumors before and after the treatment according to the categories of Ki67 LI. In pre-treatment group of the patients, androgenic enzymes including 17βHSD5, 5αRed1 and 5αRed2, were significantly correlated with each other (Table 4). Those correlations were, however, changed following AI treatment. In group 1 or whose Ki67 LI increased after the therapy, 17βHSD5 was still correlated with 5αRed2 (p = 0.009) as well as 5αRed1 with 5αRed2 (p = 0.001) but loss of correlation between 17βHSD5 and 5αRed1 was detected (p = 0.067). In group 2 or whose Ki67 LI unchanged or decreased with less than 40% of the pre-treatment level, only the correlation between 5αRed1 and 5αRed2 remained significant. The level of statistical significance was not reached in group 3 or those Ki67 LI decreased by more than 40% of the pre-treatment level (Table 4).
The Relative Importance of Androgenic Enzymes on Ki67 LI Decrement by AI Treatment
We further evaluated the effects of alterations of androgenic enzymes to determine whether these alterations, especially those of 5αRed2, were correlated with the status of response or nonresponse to the AI treatment determined by Ki67 LI changes. The status of each androgenic enzymes in post-treatment was further subclassified into three different groups according to the level of their changes after the treatment as follows: group 1; increased group, the status of the enzymes in this group was associated with an increment compared to the pre-treatment level. Group 2; no change group, the status of the enzymes was the same as that in the pre-treatment level. Group3; decreased group, the status of enzymes was decreased following the therapy. We could not find any significance among these groups in the logistic regression analysis (Table 5).
Discussion
Numerous studies have been reported on the possible roles of androgens in human breast cancer but it is also true that controversies exist as to clinical or biological significance of androgens, especially in estrogen dependent breast cancer [10–14, 17, 19, 22]. Previously, Sonne–Hansen and Lykkesfeldt reported the presence of a significant aromatase activity in the MCF-7 cells and this activity was also reported to be sufficient for the breast carcinoma cells to aromatize testosterone to estrogen, which resulted in significant cell growth stimulation [14]. In addition, both the steroidal and nonsteroidal aromatase inhibitors were reported to be able to completely abolish the growth-stimulatory effects of testosterone [14]. However, Macedo et al. reported that androgens, such as androstenedione and 5α-DHT, inhibited MCF-7 cell proliferation in a low-estrogen milieu and letrozole treatment did inhibit breast carcinoma cell proliferation by inhibiting the conversion of androgens to estrogen, and subsequently making androgens available to exert their anti-proliferative effects possibly through up-regulation of AR [10].
We also demonstrated statistically significant AR increment following the AI treatment, which is consistent with the results of previous reported studies above, but it is also true that Yamashita et al. did not detect this change during the exemestane treatment [22]. In addition, Suzuki et al. recently reported that intratumoral DHT of human breast carcinoma tissues was mainly determined by the status of 5αRed1 and aromatase [28]. In our present study, we demonstrated the correlation between the effects of AI treatment and the changes of androgenic enzymes expression. The significant correlation was also detected between the decrement in Ki67 LI or biological response of the AI treatment and the increment of 5αRed2 following AI administration in breast carcinoma patients.
Locally produced estrogens play a major role in proliferation of estrogen dependent breast cancer and androgens are considered to predominantly exert anti-proliferative effects via AR [15]. Intratumoral estrogens can be produced from circulating androgens, especially those derived from the zona reticularis of an adrenal cortex, catalyzed by the aromatase enzyme in which the neoadjuvant AI treatment blocks this enzyme with immense potency and exquisite specificity [6]. We previously demonstrated an increment of the intratumoral enzymes following AI therapy in the compensatory direction toward increasing intratumoral estrogen production [18]. However, the alteration of androgen metabolizing enzymes as a result of the neoadjuvant hormonal breast cancer therapy has not been examined at all.
Local androgen concentration has been well known to be significantly increased in breast cancer by AI treatment, as previously reported in various in vitro studies [10–12, 17, 29]. Takagi et al. recently demonstrated an increment of DHT concentration in breast carcinoma tissue following the exemestane therapy as well as the inhibitory effects of DHT on estradiol-mediated T-47D cells proliferation [19]. These findings all suggested that AI not only suppress aromatase enzyme and cause estrogen depletion in consequence, but also provide additional effects through increasing local DHT concentration, which may result in decreased cell proliferation of tumor cells. These findings were consistent with results of our present study that the statistically significant increment of 5αRed2 enzyme was detected only in the group associated with reduction of Ki67 LI with more than 40% of the pre-treatment level or group 3 (p = 0.025) (Table 3). Following AI treatment, an accumulation of in situ androgens in breast cancer tissues may occur and the enzyme 5αRed2 can serve as an important regulator of local actions of androgens because this enzyme converts testosterone into the biologically more active and nonaromatizable DHT [4, 11, 17]. However, further studies such as the analysis of much larger number of neoadjuvant treated patients are required for confirmation of this interesting hypothesis.
The potent and direct inhibitory effects of DHT on human breast cancer cell proliferation were first demonstrated by Poulin et al. in 1988 [30]. Two isoforms of 5αRed have been known to exist, encoded by different genes: SRD5A1 (chromosome 5p15) and SRD5A2 (chromosome 2p23) [31, 32]. The two types of 5αRed share 50% amino acid sequence identity and possess similar substrate specific but have different optimal pH and sensitivity to inhibitors [32]. 5αRed2 is the major form of the enzyme expressed in the human prostate [32] but rarely detected in human breast carcinoma [28]. Both Wiebe et al. [33] and Suzuki et al. [17, 28] demonstrated the expression of 5αRed1 in several types of human breast cancer cell lines using semi-quantitative RT-PCR and in human breast carcinoma tissues using IHC and RT-PCR, respectively. In addition, significant increment of 5αRed1 and 5αRed2 genes expression of human breast carcinoma as compared to normal breast tissue has been illustrated in the semi-quantitative RT-PCR study [34]. However, the regulatory mechanisms of 5αRed2 in human breast carcinoma have remained largely unknown and it awaits further investigations for clarification.
In our present study, we did not, however, detect the significant alterations in the enzymes involved in androgen metabolism in non response groups (groups 1 and 2) (Table 3). This finding suggests that androgen metabolism is not influenced by the AI treatment in these groups of patients with breast cancer or nonresponders. The loss of correlation of intratumoral androgenic enzymes in breast carcinoma tissue; 17βHSD5, 5αRed1, and 5αRed2, after AI treatment (Table 4) as well as the alterations of 5αRed1 and 5αRed2 enzymes (Table 2) were detected, but these changes did not reach statistical significance. This may be due to the relatively small size of the patients examined, especially the rather limited number of available specimens in our present study. In addition, the breast carcinoma cases associated with greater reduction of Ki67 LI tended to be associated with an increased 5αRed2, but this correlation did not reach statistical significance (Table 5).
After menopause, most of the biologically active androgens (as well as estrogens) are synthesized in peripheral intracrine tissues, for example in the breast, from precursors of adrenal origin without release of active androgens in the extracellular space and the circulation [4, 11]. In addition, DHT concentrations were demonstrated to be significantly higher in breast cancer tissues than in plasma [35]. In addition, both 17β-hydrosteroid dehydrogenase and 5α-reductases have been considered to act to increase DHT production by competing with aromatase for substrates in hormone-dependent breast carcinoma [19, 28]. As mentioned above, 5αRed1 is the predominant form of 5α-reductases at least in human breast cancer [17, 28, 32], but the results of our present study clearly demonstrate the importance of 5αRed2, which is rarely expressed in breast cancer but was increased in response group or those associated with more Ki67 decrement. We therefore hypothesized that this rather de novo 5αRed2 increment may be related to the effects of AI other than depleting in situ estrogens, i.e., the potential increment of the endogenous androgens which may exert their anti-proliferative effects via the AR, especially in a low-estrogen milieu, as demonstrated in the breast cancer cell lines study [10] and possibly to an induction in apoptosis signaling pathways. Androgens, androstenedione, and DHT, were reported to have a proapoptotic effect by strongly reducing Bcl-2 expression in MCF-7 cells, and this androgenic inhibitory effect was mediated via the AR [10, 36].
In summary, this is the first study which demonstrates an alteration of the androgen producing enzymes following the AI treatment, especially a de novo increment of 5αRed2 as well as of AR may be considered at least one of the mechanisms to account for the decreased breast carcinoma cell proliferation after AI therapy through an increment of local concentrations of androgens and their actions. However, the regulatory mechanisms of 5αRed2 in human breast carcinoma have remained largely unknown.
References
Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ (2007) Global Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society. Available at: http://www.cancer.org/docroot/STT/STT_0.asp. Accessed 19 Nov 2009
American Cancer Society (2009) Breast Cancer Facts & Figures 2009–2010. Atlanta, GA: American Cancer Society. Available at: http://www.cancer.org/docroot/STT/STT_0.asp. Accessed Nov 2009
Pasqualini JR, Chetrite GS (2005) Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J Steroid Biochem Mol Biol 93:221–236
Sasano H, Nagasaki S, Miki Y, Suzuki T (2009) New developments in intracrinology of human breast cancer estrogen sulfatase and sulfotransferase. Ann NY Acad Sci 1155:76–79
Miller WR, Forrest AP (1974) Oestradiol synthesis by a human breast carcinoma. Lancet 2:866–868
Miller WR, Anderson TJ, White S, Larionov A, Murray J, Evans D, Krause A, Dixon JM (2005) Aromatase inhibitors: cellular and molecular effects. J Steroid Biochem Mol Biol 95:83–89
Geisler J (2008) Aromatase inhibitors: from bench to bedside and back. Breast Cancer 15:17–26
Geisler J, LØnning PE (2005) Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res 11:2809–2821
Chen S, Masri S, Hong YY, Wang X, Phung S, Yuan YC, Wu X (2007) New experimental models for aromatase inhibitor resistance. J Steroid Biochem Mol Biol 106:8–15
Macedo LF, Guo Z, Tilghman SL, Sabnis GJ, Qiu Y, Brodie A (2006) Role of Androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res 66(15):7775–7782
Labrie F, Luu-The V, Labrie C, Bélanger A, Simard J, Lin SX, Pelletier G (2003) Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 24:152–182
Lippman M, Bolan G, Huff K (1976) The effect of androgens and antiandrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res 36:4610–4618
Miller WR, McDonald D, Forrest AP, Shivas AA (1973) Metabolism of androgens by human breast tissue. Lancet 1:912–913
Sonne-Hansen K, Lykkesfeldt AE (2005) Endogenous aromatization of testosterone results in growth stimulation of the human MCF-7 breast cancer cell line. J Steroid Biochem Mol Biol 93:25–34
Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H (2003) Androgen receptors frequently are expressed in breast carcinomas. Cancer 98:703–711
Sasano H, Suzuki T, Miki Y, Moriya T (2008) Intracrinology of estrogens and androgens in breast carcinoma. J Steroid Biochem Mol Biol 108:181–185
Suzuki T, Miki Y, Moriya T, Akahira J, Ishida T, Hirakawa H, Yamakuchi Y, Hayashi S, Sasano H (2006) 5α-Reductase type 1 and aromatase in breast carcinoma as regulators of in situ androgen production. Int J Cancer 120:285–291
Chanplakorn N, Chanplakorn P, Suzuki T, Ono K, Chan SMM, Miki Y, Saji S, Ueno T, Toi M, Sasano H (2010) Increased estrogen sulfatase (STS) and 17β-hydroxysteroid dehydrogenase type 1(17β-HSD1) following neoadjuvant aromatase inhibitor therapy in breast cancer patients. Breast Cancer Res Treat 120(3):639–648
Takagi K, Miki Y, Nagasaki S, Hirakawa H, Onodera Y, Akahira J, Ishida T, Watanabe M, Kimijima I, Hayashi S, Sasano H, Suzuki T (2010) Increased intratumoral androgens in human breast carcinoma following aromatase inhibitor exemestane treatment. Endocr-Relat Cancer 17(2):415–430
Chow LWC, Yip AYS, Loo WTY, Lam CK, Toi M (2008) Celecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancer. J Steroid Biochem Mol Biol 111:13–17
Miller WR, White S, Dixon JM, Murray J, Renshaw L, Anderson TJ (2006) Proliferation, steroid receptors and clinical/pathological response in breast cancer treated with letrozole. Br J Cancer 94:1051–1056
Yamashita H, Takahashi S, Ito Y, Yamashita T, Yoshiaki A, Toyama T, Sugiura H, Yoshimoto N, Kobayashi S, Fujii Y, Iwase H (2009) Predictors of response to exemestane as primary endocrine therapy in estrogen receptor-positive breast cancer. Cancer Sci 100:2028–2033
Suzuki T, Moriya T, Ariga N, Kaneko C, Kanazawa M, Sasano H (2000) 17beta-hydroxysteroid dehydrogenase type 1 and type 2 in human breast carcinoma: a correlation to clinicopathological parameters. Br J Cancer 82:518–523
Bouzubar N, Walker KJ, Griffiths K, Ellis IO, Elston CW, Robertson JFR, Blamey RW, Nicholson RI (1989) Ki67 immunostaining in primary breast cancer: pathological and clinical associations. Br J Cancer 59:943–947
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley S, Francis S, Walsh G, Smith IE (2005) Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and Her-2 in breast cancer-a study from the IMPACT trialists. J Clin Oncol 23:2477–2492
Suzuki T, Darnel AD, Akahira J, Ariga N, Ogawa S, Kaneko C, Takeyama J, Moriya T, Sasano H (2001) 5 alpha reductases in human breast carcinoma: possible modulator of in situ androgenic actions. J Clin Endocrinol Metab 86:2250–2257
Spinola PG, Marchetti B, Mérand Y, Bélanger A, Labrie F (1988) Effects of the aromatase inhibitor 4-hydroxyandrostenedione and the antiandrogen flutamide on growth and steroid levels in DMBA-induced rat mammary tumors. Breast Cancer Res Treat 12:287–296
Poulin R, Baker D, Labrie F (1988) Androgens inhibit basal and estrogen-induced cell proliferation in the ZR-75-1 human breast cancer cell line. Breast Cancer Res Treat 12:213–225
Van Gils CH, Onland-Moret C, Roest M, van Noord PAH, Peeters PHM (2003) The V89L polymorphism in the 5-α-reductase type 2 gene and risk of breast cancer. Cancer Epidemiol Biomark Prev 12:1194–1199
Van L-T, Bélanger A, Labrie F (2008) Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab 22(2):207–221
Wiebe JP, Lewis MJ, Cialacu V, Pawlak KJ, Zhang G (2005) The role of progesterone metabolites in breast cancer: potential for new diagnostics and therapeutics. J Steroid Biochem Mol Biol 93:201–208
Lewis MJ, Wiebe JP, Heathcote JG (2004) Expression of progesterone metabolizing genes (AKR1C1, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma. BMC Cancer 4:27. doi:10.1186/1471-2407/4/27
Recchione C, Venturelli E, Manzari A, Cavalleri A, Martinetti A, Secreto G (1995) Testosterone, dihydrotestosterone and oestradiol levels in postmenopausal breast cancer tissues. J Steroid Biochem Mol Biol 52:541–546
Thiantanawat A, Long BJ, Brodie AM (2003) Signaling pathways of apoptosis activated by aromatase inhibitors and antiestrogens. Cancer Res 63:8037–8050
Acknowledgment
We thank Dr. D.W. Russell (University of Texas Southwestern Medical Center, Dallas, Texas) for kindly providing antibodies against 5αRed1 and 5αRed2.
Disclosures/Conflicts of interest
Dr. Hironobu Sasano has received the educational research grant from Novartis Oncology Japan and Pfizer Oncology Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chanplakorn, N., Chanplakorn, P., Suzuki, T. et al. Increased 5α-Reductase Type 2 Expression in Human Breast Carcinoma following Aromatase Inhibitor Therapy: The Correlation with Decreased Tumor Cell Proliferation. HORM CANC 2, 73–81 (2011). https://doi.org/10.1007/s12672-010-0062-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-010-0062-2