Abstract
Purpose
While triple-negative breast cancer (TNBC) is negative for estrogen receptor alpha, a substantial proportion of carcinomas express estrogen receptor beta (ERβ); consequently, estrogen actions and metabolism may be relevant in this cancer subtype.
Methods
A cohort of 81 TNBC patients from Tohoku University Hospital, Japan were characterised with regard to the expression of estrogen receptor beta and enzymes known to modulate levels of estrogens in breast and other tissues (Aromatase, 17-beta- Hydroxysteroid dehydrogenases 1, 2 and 6). This was done at the protein level by means of immunohistochemistry. As this cohort has been previously characterised for androgens, this also allows for comparison between the expressions of estrogen-related proteins and of androgen-related proteins. Preliminary mechanistic studies in cell culture were also undertaken.
Results
17βHSD2 was detected in the highest number of cases followed by 17βHSD1, 17βHSD6 and aromatase. When comparing the expression of ERβ with that of the enzymes, it was positively correlated with the expression of 17βHSD6 (p < 0.05) and trended towards correlation with dual expression of 17βHSD1 and 2 (p < 0.07). 17βHSD1 was associated with significantly reduced tumour volume (p = 0.0025), while ERβ was associated with a trend towards reduced lymphovascular invasion, (p < 0.061). Interestingly, in survival analysis, 17βHSD6 expression was the only one of these five factors that influenced survival, with positive samples being associated with longer disease-free survival compared to those that were negative for 17βHSD6 (p < 0.05). In assessing associations with expression of proteins in the androgenic pathway, expression of aromatase appeared to be associated with androgenic pathways in TNBC patients (p < 0.05). Due to this association and the potential relevance to androgen-directed therapies in TNBC, we evaluated this interaction in vitro. We observed androgen-dependent upregulation of aromatase and ERβ in a subset of AR expressing TNBC cell lines (MDA-MB-453, SUM-185-PE and MFM-223).
Conclusion
Overall this study suggests the presence of, and a potential protective effect of estrogens in TNBC.






Similar content being viewed by others
References
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. doi:10.1093/annonc/mdt303
Marotti JD, Collins LC, Hu R, Tamimi RM (2010) Estrogen receptor-beta expression in invasive breast cancer in relation to molecular phenotype: results from the nurses’ health study. Mod Pathol 23(2):197–204. doi:10.1038/modpathol.2009.158
Guo L, Meng J, Yilamu D, Jakulin A, Fu M, Wang B, Abulajiang G (2014) Significance of ERbeta expression in different molecular subtypes of breast cancer. Diagn Pathol 9:20. doi:10.1186/1746-1596-9-20
Reese JM, Suman VJ, Subramaniam M, Wu X, Negron V, Gingery A, Pitel KS, Shah SS, Cunliffe HE, McCullough AE, Pockaj BA, Couch FJ, Olson JE, Reynolds C, Lingle WL, Spelsberg TC, Goetz MP, Ingle JN, Hawse JR (2014) ERbeta1: characterization, prognosis, and evaluation of treatment strategies in ERalpha-positive and -negative breast cancer. BMC Cancer 14(1):749. doi:10.1186/1471-2407-14-749
Wang J, Zhang C, Chen K, Tang H, Tang J, Song C, Xie X (2015) ERbeta1 inversely correlates with PTEN/PI3 K/AKT pathway and predicts a favorable prognosis in triple-negative breast cancer. Breast Cancer Res Treat. doi:10.1007/s10549-015-3467-3
Honma N, Saji S, Kurabayashi R, Aida J, Arai T, Horii R, Akiyama F, Iwase T, Harada N, Younes M, Toi M, Takubo K, Sakamoto G (2008) Oestrogen receptor-beta1 but not oestrogen receptor-betacx is of prognostic value in apocrine carcinoma of the breast. APMIS 116(10):923–930. doi:10.1111/j.1600-0463.2008.01122.x
Guo L, Zhu Q, Aisimutuola M, Yilamu D, Liu S, Jakulin A (2015) Expression and prognostic value of estrogen receptor beta in patients with triple-negative and triple-positive breast cancer. Exp Ther Med 9(6):2147–2150. doi:10.3892/etm.2015.2380
Hamilton N, Marquez-Garban D, Mah V, Fernando G, Elshimali Y, Garban H, Elashoff D, Vadgama J, Goodglick L, Pietras R (2015) Biologic roles of estrogen receptor-beta and insulin-like growth factor-2 in triple-negative breast cancer. BioMed Res Int 2015:925703. doi:10.1155/2015/925703
Wimberly H, Han G, Pinnaduwage D, Murphy LC, Yang XR, Andrulis IL, Sherman M, Figueroa J, Rimm DL (2014) ERbeta splice variant expression in four large cohorts of human breast cancer patient tumors. Breast Cancer Res Treat 146(3):657–667. doi:10.1007/s10549-014-3050-3
Smart E, Hughes T, Smith L, Speirs V (2013) Estrogen receptor beta: putting a positive into triple negative breast cancer? Horm Mol Biol Clin Investig 16(3):117–123. doi:10.1515/hmbci-2013-0042
Haldosen LA, Zhao C, Dahlman-Wright K (2014) Estrogen receptor beta in breast cancer. Mol Cell Endocrinol 382(1):665–672. doi:10.1016/j.mce.2013.08.005
Shanle EK, Zhao Z, Hawse J, Wisinski K, Keles S, Yuan M, Xu W (2013) Research resource: global identification of estrogen receptor beta target genes in triple negative breast cancer cells. Mol Endocrinol 27(10):1762–1775. doi:10.1210/me.2013-1164
Risbridger GP, Davis ID, Birrell SN, Tilley WD (2010) Breast and prostate cancer: more similar than different. Nat Rev Cancer 10(3):205–212. doi:10.1038/nrc2795
McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD (2014) Complexities of androgen receptor signalling in breast cancer. Endocr-Related Cancer 21(4):T161. doi:10.1530/erc-14-0243
Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R (2010) Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol 23(2):205–212
Tsutsumi Y (2012) Apocrine carcinoma as triple-negative breast cancer: novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol 42(5):375–386
Gatalica Z (1997) Immunohistochemical analysis of apocrine breast lesions. Consistent over-expression of androgen receptor accompanied by the loss of estrogen and progesterone receptors in apocrine metaplasia and apocrine carcinoma in situ. Pathol Res Pract 193(11–12):753–758. doi:10.1016/s0344-0338(97)80053-2
McNamara K, Yoda T, Miki Y, Chanplakorn N, Wongwaisayawan S, Incharoen P, Kongdan Y, Wang L, Takagi K, Mayu T, Nakamura Y, Suzuki T, Nemoto N, Miyashita M, Tamaki K, Ishida T, Ohuchi N, Sasano H (2013) Androgenic pathway in triple negative invasive ductal tumours: its correlation with tumour cell proliferation. Cancer Sci 104(5):639–646. doi:10.1111/cas.12121
Miyashita M, Ishida T, Ishida K, Tamaki K, Amari M, Watanabe M, Ohuchi N, Sasano H (2011) Histopathological subclassification of triple negative breast cancer using prognostic scoring system: five variables as candidates. Virchows Arch 458(1):65–72. doi:10.1007/s00428-010-1009-2
McNamara KM, Yoda T, Nurani AM, Shibahara Y, Miki Y, Wang L, Nakamura Y, Suzuki K, Yang Y, Abe E, Hirakawa H, Suzuki T, Nemoto N, Miyashita M, Tamaki K, Ishida T, Brown KA, Ohuchi N, Sasano H (2014) Androgenic pathways in the progression of triple-negative breast carcinoma: a comparison between aggressive and non-aggressive subtypes. Breast Cancer Res Treat. doi:10.1007/s10549-014-2942-6
Ariga N, Moriya T, Suzuki T, Kimura M, Ohuchi N, Satomi S, Sasano H (2000) 17 beta-Hydroxysteroid dehydrogenase type 1 and type 2 in ductal carcinoma in situ and intraductal proliferative lesions of the human breast. Anticancer Res 20(2B):1101–1108
Takagi M, Miki Y, Miyashita M, Hata S, Yoda T, Hirakawa H, Sagara Y, Rai Y, Ohi Y, Tamaki K, Ishida T, Suzuki T, Ouchi N, Sasano H (2016) Intratumoral estrogen production and actions in luminal A type invasive lobular and ductal carcinomas. Breast Cancer Res Treat 156(1):45–55. doi:10.1007/s10549-016-3739-6
Kilic Y, Celebiler AC, Sakizli M (2014) Selecting housekeeping genes as references for the normalization of quantitative PCR data in breast cancer. Clin Trans Oncol 16(2):184–190. doi:10.1007/s12094-013-1058-5
Rayoo M, Yan M, Takano EA, Bates GJ, Brown PJ, Banham AH, Fox SB (2009) Expression of the forkhead box transcription factor FOXP1 is associated with oestrogen receptor alpha, oestrogen receptor beta and improved survival in familial breast cancers. J Clin Pathol 62(10):896–902. doi:10.1136/jcp.2009.065169
Bates GJ, Fox SB, Han C, Launchbury R, Leek RD, Harris AL, Banham AH (2008) Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptor-beta in primary invasive breast carcinomas. Breast Cancer Res Treat 111(3):453–459. doi:10.1007/s10549-007-9812-4
Tan W, Li Q, Chen K, Su F, Song E, Gong C (2016) Estrogen receptor beta as a prognostic factor in breast cancer patients: a systematic review and meta-analysis. Oncotarget 7(9):10373–10385. doi:10.18632/oncotarget.7219
Lattrich C, Schuler S, Haring J, Skrzypczak M, Ortmann O, Treeck O (2014) Effects of a combined treatment with tamoxifen and estrogen receptor beta agonists on human breast cancer cell lines. Arch Gynecol Obstet 289(1):163–171. doi:10.1007/s00404-013-2977-7
Hinsche O, Girgert R, Emons G, Grundker C (2015) Estrogen receptor beta selective agonists reduce invasiveness of triple-negative breast cancer cells. Int J Oncol 46(2):878–884. doi:10.3892/ijo.2014.2778
Oduwole OO, Li Y, Isomaa VV, Mantyniemi A, Pulkka AE, Soini Y, Vihko PT (2004) 17beta-hydroxysteroid dehydrogenase type 1 is an independent prognostic marker in breast cancer. Cancer Res 64(20):7604–7609. doi:10.1158/0008-5472.can-04-0446
Weihua Z, Lathe R, Warner M, Gustafsson JA (2002) An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci USA 99(21):13589–13594
Honma N, Saji S, Hirose M, Horiguchi S, Kuroi K, Hayashi S, Utsumi T, Harada N (2011) Sex steroid hormones in pairs of tumor and serum from breast cancer patients and pathobiological role of androstene-3beta, 17beta-diol. Cancer Sci 102(10):1848–1854. doi:10.1111/j.1349-7006.2011.02018.x
Yoda T, Kikuchi K, Miki Y, Onodera Y, Hata S, Takagi K, Nakamura Y, Hirakawa H, Ishida T, Suzuki T, Ohuchi N, Sasano H, McNamara KM (2015) 11beta-Prostaglandin F2alpha, a bioactive metabolite catalyzed by AKR1C3, stimulates prostaglandin F receptor and induces slug expression in breast cancer. Mol Cell Endocrinol. doi:10.1016/j.mce.2015.07.008
McNamara KM, Yoda T, Miki Y, Nakamura Y, Suzuki T, Nemoto N, Miyashita M, Nishimura R, Arima N, Tamaki K, Ishida T, Ohuchi N, Sasano H (2014) Androgen receptor and enzymes in lymph node metastasis and cancer reoccurrence in triple-negative breast cancer. Int J Biol Mark. doi:10.5301/jbm.5000132
Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE (1994) Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology 135(1):395–401
Maris P, Campana A, Barone I, Giordano C, Morelli C, Malivindi R, Sisci D, Aquila S, Rago V, Bonofiglio D, Catalano S, Lanzino M, Ando S (2015) Androgens inhibit aromatase expression through DAX-1: insights into the molecular link between hormone balance and Leydig cancer development. Endocrinology 156(4):1251–1262. doi:10.1210/en.2014-1654
Rizza P, Barone I, Zito D, Giordano F, Lanzino M, De Amicis F, Mauro L, Sisci D, Catalano S, Wright KD, Gustafsson JA, Ando S (2014) Estrogen receptor beta as a novel target of androgen receptor action in breast cancer cell lines. Breast Cancer Res 16(1):R21. doi:10.1186/bcr3619
Hackenberg R, Luttchens S, Hofmann J, Kunzmann R, Holzel F, Schulz KD (1991) Androgen sensitivity of the new human breast cancer cell line MFM-223. Cancer Res 51(20):5722–5727
Hickey TE, Robinson JL, Carroll JS, Tilley WD (2012) Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol 26(8):1252–1267
Acknowledgements
The authors would like to acknowledge all the members of their laboratories, whose informal input was extremely valuable. The authors would like to acknowledge the extremely capable assistance provided by both Katsuhiko Ono and Yoshiaki Onodera, in particular, and more generally by the staff at the Anatomical Pathology department of the Tohoku University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2016_4050_MOESM2_ESM.pdf
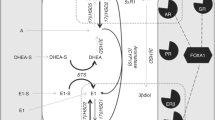
Supplementary material 2 (PDF 307 kb) Figure S1 Potential pathways of estrogen metabolism in TNBC. These figures show the enzymatic conversion of steroids (black lines) alongside the predominant or only enzymes known to catalyse that reaction (white box on top of black line). In addition, steroids as potential ligands of receptors are shown(grey dotted lines). While we and others have previously studied androgen metabolism pathways in detail (5αR1, 17βHSD5) estrogen metabolism pathways have been relatively neglected, even though these are only one enzymatic step from androgens. The presence of ERβ in TNBC tissue provides an important rationale for studying these enzymes. It should also be noted as in the arrows at the right that the presence or absence of these enzymes can determine if a carcinoma is androgen dominant or estrogen dominant. Estrogen metabolism will reduce the pool of circulating androgens and likewise androgen metabolism will limit the pool of circulating estrogens, thus these two pathways interact on multiple levels. Two pathways of estrogen metabolism are shown. In red, the canonical pathway of estrogen synthesis is given involving the non-reversible aromatization of C19 steroids to C18 steroids with the use of relatively low-potency androgens as estrogenic precursors. In blue the 3β-diol pathway is highlighted with the use of the most-potent androgen, DHT as a precursor to the production of an ERβ-selective C19 steroid, 3β-diol
10549_2016_4050_MOESM3_ESM.pdf
Supplementary material 3 (PDF 455 kb) Figure S2 Changes in expression between Normal, DCIS and IDC. IHC of histologically normal adjacent to the DCIS shown here is given in A–D. IHC immunoreactivity of ERβ1, aromatase and 17βHSD6 in DCIS is shown (E–H). Based on changes observed between normal and cancerous and DCIS and IDC components we examined the changes in expression of ERβ (I), aromatase (J) and 17βHSD6 (K) between TNBC DCIS and the levels observed in the IDC samples. In this analysis, we noted a significant decrease in the expression of 17βHSD6 (p < 0.001, Chi squared Pearsons) with no other significant changes observed on the basis of histology. The scale bar represents 200 μM
Rights and permissions
About this article
Cite this article
McNamara, K.M., Oguro, S., Omata, F. et al. The presence and impact of estrogen metabolism on the biology of triple-negative breast cancer. Breast Cancer Res Treat 161, 213–227 (2017). https://doi.org/10.1007/s10549-016-4050-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4050-2