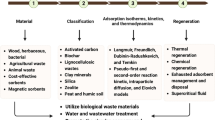
Abstract
The removal of free cyanide from aqueous solutions was investigated by kaolin, calcined kaolin (metakaolin), and acid-treated metakaolin. The kaolin in raw form had no ability for cyanide adsorption, but calcination and acid treatment significantly improve its adsorptive properties. The best calcination conditions were determined to be at temperature of 600 °C and calcination time of 1 h. The scanning electron microscope (SEM), wavelength-dispersive X-ray (WDX), X-ray fluorescence (XRF), and X-ray diffraction (XRD) analysis methods were used to explain the adsorptive properties of the adsorbents. The loading capacity of metakaolin increased from 0.66 to 5.32 mg/g, and the loading capacity of acid-treated metakaolin increased from 1.33 to 10.67 mg/g by increasing the initial cyanide concentration from 25 to 531 mg/L. The kinetics of cyanide adsorption onto both adsorbents increases by increasing the initial cyanide concentration (25–531 mg/L) and solution temperature (30–50 °C). The equilibrium and kinetic data on both adsorbents are best fitted by the Freundlich isotherm and pseudo-second-order kinetic models, respectively. The thermodynamic study showed that the cyanide adsorption onto both adsorbents is endothermic and spontaneous in nature. The activation energy for the cyanide adsorption onto metakaolin and acid-treated metakaolin was determined to be 16.68 and 5.79 kJ/mol which indicates that the adsorption of cyanide ions onto metakaolin is chemisorption and onto the acid-treated metakaolin is a physicochemical process.









Similar content being viewed by others
References
Abdalla OAE, Suliman FO, Al-Ajmi H, Al-Hosni T, Rollinson H (2010) Cyanide from gold mining and its effect on groundwater in arid areas, Yanqul mine of Oman. Environ Earth Sci 60(4):885–892
Abdel-Karim AM, Zaki AA, Elwan W, El-Naggar MR, Gouda MM (2016) Experimental and modeling investigations of cesium and strontium adsorption onto clay of radioactive waste disposal. Appl Clay Sci 132–133:391–401
Abdel-Karim AM, Zaki AA, Elwan W, El-Naggar MR, Gouda MM (2019) Geological and contaminant transport assessment of a low level radioactive waste disposal site. J Geochem Explor 197:174–183
Abdullahi Y, Ali EA, Lawal AO (2013) Roast alkaline leaching of silica from kaolinitic clay. ARPN J Eng Appl Sci 8(10):864–870
Aghdasinia H, Asiabi RH (2018) Adsorption of a cationic dye (methylene blue) by Iranian natural clays from aqueous solutions: equilibrium, kinetic and thermodynamic study. Environ Earth Sci 77:218
Anna B, Kleopas M, Constantine S, Anestis F, Maria B (2015) Adsorption of Cd(II), Cu(II), Ni(II) and Pb(II) onto natural bentonite: study in mono-and multi-metal systems. Environ Earth Sci 73(9):5435–5444
Banat FA, Al-Bashir B, Al-Asheh S, Hayajneh O (2000) Adsorption of phenol by bentonite. Environ Pollut 107(3):391–398
Barkat M, Nibou D, Chegrouche S, Mellah A (2009) Kinetics and thermodynamics studies of chromium(VI) ions adsorption onto activated carbon from aqueous solutions. Chem Eng Process 48(1):38–47
Behnamfard A, Salarirad MM (2009) Equilibrium and kinetic studies on free cyanide adsorption from aqueous solution by activated carbon. J Hazard Mater 170(1):127–133
Behnamfard A, Salarirad MM (2014) Characterization of coconut shell-based activated carbon and its application in the removal of Zn(II) from its aqueous solution by adsorption. Desalin Water Treat 52(37–39):7180–7195
Chen NC, Chen D (2005) Mullite Composite phase nanocrystalline from high-silica kaolin under normal pressure and temperature: an experimental study. Key engineering materials, vol 280. Trans Tech Publications, Amsterdam, pp 1157–1160
De Mesquita LMS, Rodrigues T, Gomes SDS (1996) Bleaching of Brazilian kaolins using organic acids and fermented medium. Miner Eng 9(9):965–971
Deng HM, Chen YH, Wu HH, Liu T, Wang YL, Wu GY, Ye HP (2016) Adsorption of Tl(I) on Na–montmorillonite and kaolinite from aqueous solutions. Environ Earth Sci 75(9):752
Drzal LT, Rynd JP, Fort T Jr (1983) Effects of calcination on the surface properties of kaolinite. J Colloid Interface Sci 93(1):126–139
Dwivedi N, Balomajumder C, Mondal P (2016) Comparative investigation on the removal of cyanide from aqueous solution using two different bioadsorbents. Water Resour Ind 15:28–40
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10
Foo CT, Mahmood CS, Salleh MAM (2011) The study of aluminum loss and consequent phase transformation in heat-treated acid-leached kaolin. Mater Charact 62(4):373–377
Kim M, Hyun S, Sung JS (2013) Adsorptive attenuation of ferrocyanide from seepage water in landfill clay liners. Environ Earth Sci 68(7):2007–2014
Lafi AAHF, Matam SK, Hodali HA (2015) New synthesis of ZSM-5 from high-silica Kaolinite and its use in vapor-phase conversion of 1-phenylethanol to styrene. Ind Eng Chem Res 54(15):3754–3760
Laus R, Costa TG, Szpoganicz B, Fávere VT (2010) Adsorption and desorption of Cu (II), Cd (II) and Pb(II) ions using chitosan crosslinked with epichlorohydrin-triphosphate as the adsorbent. J Hazard Mater 183(1–3):233–241
Liu Q, Zhang G, Ding J, Zou H, Shi H, Huang C (2018) Evaluation of the removal of potassium cyanide and its toxicity in green algae (Chlorella vulgaris). Bull Environ Contam Toxicol 100(2):228–233
Ma J, Dasgupta PK (2010) Recent developments in cyanide detection: a review. Anal Chim Acta 673(2):117–125
Makó É, Senkár Z, Kristóf J, Vágvölgyi V (2006) Surface modification of mechanochemically activated kaolinites by selective leaching. J Colloid Interface Sci 294(2):362–370
Maulana I, Takahashi F (2018) Cyanide removal study by raw and iron-modified synthetic zeolites in batch adsorption experiments. J Water Process Eng 22:80–86
Moussavi G, Khosravi R (2010) Removal of cyanide from wastewater by adsorption onto pistachio hull wastes: parametric experiments, kinetics and equilibrium analysis. J Hazard Mater 183(1):724–730
Murray HH (1991) Overview—clay mineral applications. Appl Clay Sci 5(5–6):379–395
Murray HH (2000) Traditional and new applications for kaolin, smectite, and palygorskite: a general overview. Appl Clay Sci 17(5–6):207–221
Murray HH (2006) Chapter 5 Kaolin applications. In: Murray HH (ed) Developments in clay science. Elsevier, Amsterdam, pp 85–109
Noroozi R, Al-Musawi TJ, Kazemian H, Kalhori EM, Zarrabi M (2018) Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. J Water Process Eng 21:44–51
Özacar M, Şengil IA (2003) Adsorption of reactive dyes on calcined alunite from aqueous solutions. J Hazard Mater 98(1–3):211–224
Panda AK, Mishra BG, Mishra DK, Singh RK (2010) Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surf A Physicochem Eng Asp 363(1–3):98–104
Pinna EG, Suarez DS, Rosales GD, Rodriguez MH (2017) Hydrometallurgical extraction of Al and Si from kaolinitic clays. REM Int Eng J 70(4):451–457
Pirmoradi M, Hashemian S, Shayesteh MR (2017) Kinetics and thermodynamics of cyanide removal by ZnO@NiO nanocrystals. Trans Nonferrous Met Soc China 27(6):1394–1403
Ptáček P, Kubátová D, Havlica J, Brandštetr J, Šoukal F, Opravil T (2010) The non-isothermal kinetic analysis of the thermal decomposition of kaolinite by thermogravimetric analysis. Powder Technol 204(2–3):222–227
Qiu G, Jiang T, Li G, Fan X, Huang Z (2004) Activation and removal of silicon in kaolinite by thermochemical process. Scand J Metall 33(2):121–128
Rani RD, Sasidhar P (2012) Geochemical and thermodynamic aspects of sorption of strontium on kaolinite dominated clay samples at Kalpakkam. Environ Earth Sci 65(4):1265–1274
Rivera O, Pavez O, Kao JL, Nazer A (2016) Metallurgical characterization of kaolin from Atacama, Chile. REM Inter Eng J 69(4):473–478
Rizzo L, Meric S, Guida M, Kassinos D, Belgiorno V (2009) Heterogenous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res 43(16):4070–4078
Saikia NJ, Bharali DJ, Sengupta P, Bordoloi D, Goswamee RL, Saikia PC, Borthakur PC (2003) Characterization, beneficiation and utilization of a kaolinite clay from Assam, India. Appl Clay Sci 24(1–2):93–103
Sim JH, Seo HJ, Kim CG (2009) Physicochemical characteristics for adsorption of MTBE and cadmium on clay minerals. Environ Earth Sci 59(3):537–545
Tironi A, Trezza MA, Irassar EF, Scian AN (2012) Thermal treatment of kaolin: effect on the pozzolanic activity. Procedia Mater Sci 1:343–350
Tsai WT, Chang CY, Ing CH, Chang CF (2004) Adsorption of acid dyes from aqueous solution on activated bleaching earth. J Colloid Interface Sci 275(1):72–78
Tyagi M, Rana A, Kumari S, Jagadevan S (2018) Adsorptive removal of cyanide from coke oven wastewater onto zero-valent iron: optimization through response surface methodology, isotherm and kinetic studies. J Clean Prod 178:398–407
Xi J, He M, Kong L (2016) Adsorption of antimony on kaolinite as a function of time, pH, HA and competitive anions. Environ Earth Sci 75:136
Zheng W, Wang Y, Yang L, Li X, Zhou L, Li Y (2014) Novel adsorbent of polymeric complex derived from chaleting resin with Cu(II) and its removal properties for cyanide in aqueous solution. Colloids Surf A Physicochem Eng Asp 455:136–146
Acknowledgements
The authors of this paper express their deep gratitude to the research and technology affairs of University of Birjand for providing some of the project costs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Behnamfard, A., Chegni, K., Alaei, R. et al. The effect of thermal and acid treatment of kaolin on its ability for cyanide removal from aqueous solutions. Environ Earth Sci 78, 408 (2019). https://doi.org/10.1007/s12665-019-8408-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8408-8