Abstract
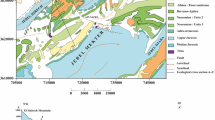
Origins and processes of groundwater salinization and pollution in urban coastal aquifer under the influence of serious industrialization and urbanization were determined using major and trace element/ion geochemistry, microbial and environmental isotope analyses, and statistical correlation and hierarchical cluster analyses of chemical parameters. Electrical conductivities (EC) of the groundwater in the coastal aquifer ranged from 487 to 4280 µS/cm. Average groundwater EC values measured in wet and dry seasons were 1130 ± 420 µS/cm and 1096 ± 526 µS/cm, respectively. Wells with high EC values were noticed not only near the gulf coastline (1799–2801 µS/cm) but also at the further inland (1709–4280 µS/cm). Statistical evaluation of the groundwater analyses showed that EC had a relatively good correlation with Cl (r = 0.85–0.90, p < 0.01). Cl–Br ion couple also exhibited a significant correlation with a r squared value of 0.9545, suggesting that salinity of the coastal aquifer was controlled dominantly by single source. Piper diagram showed that predominant cation and anion types in the coastal groundwater exhibited shifts generally from Ca to Na, HCO3 to Cl and to less HCO3 to SO4. A significant variation observed in hydrogeochemical facies of the coastal groundwater resulted from dissolution of calcite, dolomite and gypsum, mixing with seawater, formation water and sewage effluents, and cation-exchange processes. Evaluation of Cl/Br mass ratio along with total nitrogen and microbiological contents of the groundwater samples indicated that seawater intrusion was occurring in the coastal aquifer. However, other sources such as mixing with saline formation water (paleo-seawater) and sewage effluents also contributed groundwater salinization locally at the inland. High seawater mixing ratios (2.5–5%) were obtained from wells close to the gulf coastline, while inland wells generally exhibited low mixing ratios (mean 0.36% ± 0.3). Fecal contamination in coastal groundwater was at notable levels. Varying redox environments (oxic/suboxic/anoxic) were observed in the coastal aquifer. Seasonal variation in the redox conditions controlled the Mn, Fe, and As enrichments in the coastal groundwater above their MCL values. This study also showed when employed with the other pollutant indicators, Cl/Br mass ratio could be used effectively to delineate the sources of contamination and salinization in coastal groundwater exhibiting low seawater mixing ratios.















Similar content being viewed by others
References
Abdalla F (2016) Ionic ratios as tracers to assess seawater intrusion and to identify salinity sources in Jazan coastal aquifer, Saudi Arabia. Arab J Geosci 9:40. https://doi.org/10.1007/s12517-015-2065-3
Akouvi A, Dray M, Violette S, De Marsily G, Zuppi GM (2008) The sedimentary coastal basin of Togo: example of a multilayered aquifer still influenced by a paleo-seawater intrusion. Hydrogeol J 16:419–436
Anders R, Mendez GO, Futa K, Danskin WR (2014) A geochemical approach to determine sources and movement of saline groundwater in a coastal aquifer. Ground water 52(5):756–768
Appelo CAJ, Postma D (1993) Geochemistry, groundwater and pollution. A. A. Balkema, Rotterdam, 536 pp
Barbecot F, Marlin C, Gibert E, Dever L (2000) Hydrochemical and isotopic characterization of the Bathonian and Bajocian coastal aquifer of the Caen area (northern France). Appl Geochem 15:791–805
Bear J, Cheng AH, Sorek S, Ouazar D, Herrers I (1999) Seawater Intrusion in Coastal Aquifer Concepts, Methods, and Practices. Kluwer Academic Publishers, London
Behrends T, Bruggeman C (2016) Determining redox properties of clay-rich sedimentary deposits in the context of performance assessment of radioactive waste repositories: conceptual and practical aspects. OPERA-PU-UTR611-1, p 29
Cartwright I, Weaver TR, Fulton S, Nichol C, Reid M, Cheng X (2004) Hydrogeochemical and isotopic constrains on the origins of dryland salinity, Murray basin, Victoria, Australia. Appl Geochem 19:1233–1254
Clark ID, Fritz P (1997) Environmental Isotopes in hydrogeology. Lewis Publishers, Boca Raton
Colombani N, Volta G, Osti A, Mastrocicco M (2016) Misleading reconstruction of seawater intrusion via integral depth sampling. J Hydrol 536:320–326
Davis SN, Whittemore DO, Fabryka-Martin J (1998) Uses of chloride/bromide ratios in studies of potable water. Water Resour Res 36(2):338–350
De Montety V, Radakovitch O, Vallet-Coulomb C, Blavoux B, Hermitte D, Valles V (2008) Origin of groundwater salinity and hydrogeochemical processes in a confined coastal aquifer: case of the Rhone delta (Southern France). Appl Geochem 23(8):2337–2349
DSI (1963) İzmit-Sapanca-Gölcük civarının hidrojeolojik etüd raporu, T.C. Enerji ve Tabii Kaynaklar Bakanlığı Devlet Su İşleri Genel Müdürlüğü (In Turkish), pp 1–30
Dubrovsky NM, Burow KR, Clark GM, Gronberg JM, Hamilton PA, Hitt KJ, Mueller DK, Munn MD, Nolan BT, Puckett LJ, Rupert MG, Short TM, Spahr NE, Sprague LA, Wilber WG (2010) The quality of our Nation’s waters—nutrients in the nation’s streams and groundwater, 1992–2004: U.S. Geological Survey Circular 1350
Fakir Y, Mernissi ME, Kreuser T, Berjami B (2002) Natural tracer approach to characterize groundwater in the coastal Sahel of Oualidia (Morocco). Environ Geol 43:197–202
Farid I, Zouari K, Rigane A, Beji R (2015) Origin of the groundwater salinity and geochemical processes in detrital and carbonate aquifers: case of Chougafiya basin (Central Tunisia). J Hydrol 508–532
Faye SP, Maloszewski W, Stichler P, Trimborn SC, Gaye CB (2005) Groundwater salinization in the Saloum (Senegal) delta aquifer: minor elements and isotopic indicators. Sci Total Environ 343:243–259
Ferchichi H, Ben Hamouda MF, Farhat B, Ben Mammou A (2018) Assessment of groundwater salinity using GIS and multivariate statistics in a coastal Mediterranean aquifer. Int J Environ Sci Technol 15:2473. doi.https://doi.org/10.1007/s13762-018-1767-y
Fidelibus MD (2003) Environmental tracing in coastal aquifers: old problems and new solutions. In: Coastal aquifers intrusion technology: Mediterranean countries, vol II. Publ. IGME, Madrid, pp 79–111
Fidelibus MD, Calò G, Tinelli R, Tulipano L (2011) Salt ground waters in the Salento karstic coastal aquifer (Apulia, Southern Italy). In: Lambrakis N, Stournaras G, Katsanou K (eds), Advances in the research of aquatic environment, environmental earth sciences series, vol 1. Springer-Verlag, Berlin, pp 407–415
Ghabayen SMS, McKee M, Kemblowski M (2006) Ionic and isotopic ratios for identification of salinity sources and missing data in the Gaza aquifer. J Hydrol 318(1–4):360–373
Ghassemi F, Jakeman AJ, Nix HA (1995) Salinization of land and water resources. Human causes, extent, management and case studies. CAB International, Wallingford, p 526
Giambastiani BMS, Colombani N, Mastrocicco M (2013) Characterization of the lowland coastal aquifer of Comacchio (Ferrara, Italy): hydrology, hydrochemistry and evolution of the system. J Hydrol 50:35–44
Gimenez Forcada E, Morell Evangelista I (2008) Contributions of boron isotopes to understanding the hydrogeochemistry of the coastal detritic aquifer of Castellòn Plain, Spain. Hydrogeol J 16:547–557
Granato GE (1996) Deicing chemicals as source of constituents of highway runoff. Transp Res Rec J Transp Res Board. https://doi.org/10.1177/0361198196153300108
Hem JD (1992) Study and interpretation of the chemical characteristics of natural water, 3rd edn. US Geological Survey Water Supply Paper 2254, 263
Hudak PF (2003) Chloride/bromide ratios in leachate derived from farm-animal waste. Environ Poll 121:23–25
Katz BG, Eberts SM, Kauffman LJ (2011) Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems: a review and examples from principal aquifers in the United States. J Hydrol 397:151–166
Kelly F (2005) Seawater Intrusion topic paper (final), vol 6. WRIA, Island County, pp 1–30
Khaska M, La Salle CL, Lancelot J, Aster team, Mohammad A, Verdoux P, Noret A, Simler R (2013) Origin of groundwater salinity (current seawater vs. saline deep water) in coastal karst aquifer based on Sr and Cl isotopes. Case study of the La Clape massif (southern France). Appl Geochem 37:212–227
Kim KH, Yun ST, Park SS, Joo Y, Kim TS (2014) Model-based clustering of hydrochemical data to demarcate natural versus human impacts on bedrock groundwater quality in rural areas. South Korea J Hydrol 519:626–636
Krauskopf KB (1979) Introduction to geochemistry. McGraw-Hill, New York, p 721
Panno SV, Hackley KC, Hwang HH, Greenberg SE, Krapac IG, Landsberger S, O’Kelly DJ (2005) Data table for characterization and identification of the sources of Na- Cl in natural waters. Illinois State Geological Survey Open File Series 2005–1. Champaign
Panno SV, Hackley KC, Hwang HH, Greenberg SE, Krapac IG, Landsberger S, O’Kelly DJ (2006) Characterization and Identification of Na–Cl sources in ground water. Ground water 44(2):176–187
Parkhurst DL (1995) User’s guide to PHREEQC—A computer program for speciation, reaction-path, advective-transport, and inverse geochemical calculations. US Geological Survey, Water-Resources Investigations Report 95-4227, pp 1–143
Sacks LA, Tihansky AB (1996) Geochemical and isotopic composition of ground water, with emphasis on sources of sulfate, in the Upper Floridan Aquifer and Intermediate Aquifer System in Southwest Florida. US Department of the Interior, US Geological Survey, Water Resources Investigations Report 96-4146, p 59
Sanchez Martos F, Pulido Bosch A, Calaforra JM (1998) Hydrogeochemical processes in arid region of Europe (Almeria, SE Spain). Appl Geochem. 14: 735–745
Sanchez-Martos F, Pulido-Bosch A, Molina-Sanchez L, Vallejos-Izquierdo A (2002) Identification of the origin of salinization in groundwater using minor ions (Lower Andarax, Southeast Spain). Sci Total Environ 297:43–58
Schoonen MAA, Devoe V, Brown CJ (1995) Bromide in Long Island ground waters and surface waters. In: Geology of long Island and Metropolitan New York, April 22, 1995, program with abstracts. Stony Brook, New York, pp 118–126
Somay MA, Gemici U (2009) Assessment of the salinization process at the coastal area with hydrogeochemical tools and geographical information systems (GIS): Selcuk Plain, Izmir, Turkey. Water Air Soil Pollut 201(1–4):55–74
Timur E, Aksay A (2002) Adapazarı G24 paftasının 1.100.000 ölçekli jeolojik etüdü, Maden Tetkik ve Arama (MTA), 31:19
Vengosh A, Gill J, Davisson ML, Hudson GB (2002) A multi-isotope (B, Sr, O, H, and C) and age dating (3H,3He and 14C) study of groundwater from Salinas Valley, California: hydrochemistry, dynamics, and contamination processes. Water Resour Res 38(1):1008. https://doi.org/10.1029/2001WR000517
Walraevens K, Cardenal J, Van Camp M (2007) Reaction transport modelling of a freshening aquifer (Tertiary Ledo-Paniselian Aquifer, Flanders-Belgium). Appl Geochem 22:289–305
Walter J, Chesnaux R, Cloutier V, Gaboury D (2017) The influence of water/rock-water/clay interactions and mixing in the salinization processes of groundwater. J Hydrol 13:168–188
Wang Y, Jiao JJ (2012) Origin of groundwater salinity and hydrogeochemical processes in the confined quaternary aquifer of the Pearl River Delta, China. J Hydrol 438–439:112–124
Webb MT, Howard KWF (2011) Modeling the transient response of saline intrusion to rising sea-levels. Ground water 49(4):560–569
Yamanaka M, Kumagai Y (2006) Sulfur isotope constraint on the provenance of salinity in a confined aquifer system of the southwestern Nobi Plain, central Japan. J Hydrol 325(1–4):35–55
Acknowledgements
This study was funded by Kocaeli Water and Sewerage Department. We thank Ercan Sanğu and Ahmet Şener for their helps on drawing some illustrations. We also thank anonymous reviewers for their constructive and valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yolcubal, İ., Ataş Gündüz, Ö.C. & Kurtuluş, N. Origin of salinization and pollution sources and geochemical processes in urban coastal aquifer (Kocaeli, NW Turkey). Environ Earth Sci 78, 181 (2019). https://doi.org/10.1007/s12665-019-8181-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8181-8