Abstract
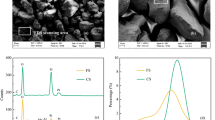
This article presents the effect of phosphate ion on the potential leaching of arsenic from the contaminated sediment under anaerobic conditions near neutral pH for 120 h. Arsenic content in the selected sediments was 450 mg/kg. In the leaching experiment, 50 g of sediment sample was added into 400 mL of five different concentrated 0 (no phosphate), 3, 10, 15, and 20 mg/L phosphate solutions, and leachates were analyzed by distinguishing As(III) and As(V) with the sequential hydride generation flow injection analyzer (SHGFIA). The results indicate that arsenic concentration in the leachates was increased for As(V) up to 72 h, and subsequently As(V) concentration was decreased in the suspension. The leached amount of As(V) concentration in the aqueous phase was increased with increasing the phosphate concentration in the suspensions, and statistically significant correlation (R 2 = 0.9959) was found. Conversely, As(III) concentration in the leachates was almost constant up to 24 h then As(III) concentration was decreased in the suspension. The phosphate ion was potentially leached arsenic, especially As(V) as phosphate (PO4 3−) ion has more charge density than arsenate (AsO4 3−) resulting more As(V) was leached from the contaminated sediment with increasing the phosphate concentration in the suspensions.





Similar content being viewed by others
References
Acharyya SK, Chakraborty P, Lahiri S, Raymahashay BC, Guha S, Bhowmik A (1999) Arsenic poisoning in the Ganges delta. Nature 401:545–546
Acharyya SK, Lahiri S, Ramahashay BC, Bowmik A (2000) Arsenic toxicity of groundwater in parts of the Bengal basin in India and Bangladesh: the role of Quaternary stratigraphy and Holocene sea-level fluctuation. Environ Geol 39(10):1127–1137
Akter KF, Chen Z, Smith L, Davey D, Naidu R (2005) Speciation of arsenic in ground water samples: a comparative study of CE-UV, HG-AAS and LC-ICP-MS. Talanta 68:406–415
Anawar HM, Akai J, Komaki K, Terao H, Yoshioka T, Ishizuka T, Safiullah S, Kato K (2003) Geochemical occurrence of arsenic in groundwater of Bangladesh: sources and mobilization processes. J Geochem Explor 77:109–131
Bagla P, Kaiser J (1996) India’s spreading health crisis draws global arsenic experts. Science 74:174–175
Berg M, Tran HC, Guyen TC, Pham HV, Schertenleib R, Giger W (2001) Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat. Environ Sci Technol 35(13):2621–2626
Carbonell-Barrachina AA, Jugsujinda A, Burlo F, Delaune RD, Patrick JRWH (1999) Arsenic chemistry in municipal sewage sludge as affected by redox potential and pH. Water Res 34:216–224
Chakraborti D, Rahman MM, Paul K, Chowdhury UK, Sengupta MK, Lodh D, Chanda CR, Saha KC, Mukherjee SC (2002) Arsenic calamity in the Indian subcontinent, what lessons have been learned? Talanta 58:3–22
Dixit S, Hering JG (2003) Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol 37(18):4182–4189
Ettler V, Komarkova M, Jehlicka J, Coufal P, Hradil D, Machovic V, Delorme F (2004) Leaching of lead metallurgical slag in citric solutions-implications for disposal and weathering in soil environments. Chemosphere 57:567–577
Fazal MA, Kawachi T, Ichion E (2001) Extend and severity of groundwater arsenic contamnation in bangladesh. Water Int 26(3):370–379
Fedotov PS, Fitz WJ, Wennrich R, Morgenstern P, Wenzel WW (2005) Fractionation of arsenic in soil and sludge samples: continuous-flow extraction using rotating coiled columns versus batch sequential extraction. Anal Chim Acta 538:93–98
Hashem MA, Takaki M, Toda K (2011) Measurements of arsenite and arsenate contained in mining river waters and leached from contaminated sediments by sequential hydride generation flow injection analysis. Talanta 84:1336–1341
Igarashi T, Imagawa H, Uchiyama H, Asakura K (2008) Leaching behavior of arsenic from various rocks by controlling geochemical conditions. Miner Eng 21:191–199
Jacob DL, Otte ML (2004) Influence of Typha latifolia and fertilization on metal mobility in two different Pb–Zn mine tailings types. Sci Total Environ 333:9–24
Jain A, Loeppert RH (2000) Effect of competing anions on the adsorption of arsenate and arsenite by ferrihydrite. J Environ Qual 29:1422–1430
Kenkyukai Kagaku Jikken Tekisuto (ed) (1997) Heavy metals in sediments (pretreatments) (in Japanese), Kankyo Kagaku. Sangyo Tosho Co., Tokyo, p 66
Kinniburgh DG, Kosmus W (2002) Arsenic contamination in groundwater: some analytical considerations. Talanta 58:165–180
Krysiak A, Karczewska A (2007) Arsenic extractability in soils in the areas of former arsenic mining and smelting, SW Poland. Sci Total Environ 379:190–200
Kumaresan M, Riyazuddin P (2001) Overview of speciation chemistry of arsenic. Curr Sci 80(7):837–846
Lombi E, Sletten RS, Wenzel WW (2000) Sequentially extracted arsenic from different size fractions of contaminated soils. Water Air Soil Poll 124:319–332
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Masue Y, Loeppert RH, Kramer TA (2007) Arsenate and arsenite adsorption and desorption behavior on Coprecipitated aluminum: iron hydroxides. Environ Sci Technol 41:837–842
Meng X, Korfiatis GP, Jing C, Christodoulatos C (2001) Redox transformations of arsenic and iron in water treatment sludge during aging and TCLP extraction. Environ Sci Technol 35:3476–3481
Minakata K, Suzuki M, Suzuki O (2009) Simple and selective determination of arsenite and arsenate by electrospray ionization mass spectrometry. Anal Chim Acta 631(1):87–90
Miro M, Hansen EH, Chomchoei R, Frenzel W (2005) Dynamic flow-through approaches for metal fractionation in environmentally relevant solid samples. Trends Anal Chem 24(8):759–771
Muller J (1999) Determination of inorganic arsenic(III) in ground water using generation coupled to ICP-AES (HG–ICP–AES) under various sodium boron hydride (NaBH4) concentrations. Fresenius J Anal Chem 363:572–576
National Research Council, Arsenic in Drinking Water (1999 and 2001 update) National Academy Press, Washington, DC
Nickson RT, McArthur JM, Ravenscroft P, Burgess WG, Ahmed KM (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
Nordstrom DK (2002) Worldwide arsenic occurrence of Arsenic in ground water. Science 296:2143–2145
Noubactep C (2012) Characterising the reactivity of metallic iron in Fe0/As-rock/H2O systems by long-term column experiments. Water SA 38(4):511–518
Noubactep C, Chen-Braucher D, Schlothauer T (2008) Arsenic release from a natural rock under near-natural oxidizing conditions. Eng Life Sci 8(6):622–630
Rigol A, Mateu J, Gonzalez-Nunez R, Rauret G, Vidal M (2009) pHstat vs. single extraction tests to evaluate heavy metals and arsenic leachability in environmental samples. Anal Chim Acta 632:69–79
Roussel C, Neel C, Bril H (2000) Minerals controlling arsenic and lead solubility in an abandoned gold mine tailings. Sci Total Environ 263:209–219
Sengupta MK, Dasgupta PK (2009) An automated hydride generation interface to ICPMS for measuring total arsenic in environmental samples. Anal Chem 81:9737–9743
Smith E, Naidu R, Alston AM (2002) Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorption. J Environ Qual 31:557–563
Stoltz E, Greger M (2006) Release of metals and arsenic from various mine tailings by Eriophorum angustifolium. Plant Soil 289:199–210
Tabelin CB, Igarashi T (2009) Mechanisms of arsenic and lead release from hydrothermally altered rock. J Hazard Mater 169:980–990
Toda K, Ohba T, Takaki M, Karthikeyan S, Hirata S, Dasgupta PK (2005) Speciation-capable field instrument for the measurement of arsenite and arsenate in water. Anal Chem 77:4765–4773
Toda K, Takaki M, Hashem MA (2008) Investigation of arsenic removal in batch wise water treatments by means of sequential hydride generation flow injection analysis. Chemosphere 72:1517–1523
Violante A, Pigna M (2002) Competitive sorption of arsenate and phosphate on different clay minerals and soils. Soil Sci Soc Am J 66:1788–1796
Wei C, Liu J (2007) A new hydride generation system applied in determination of arsenic species with ion chromatography–hydride generation-atomic fluorescence spectrometry (IC-HG-AFS). Talanta 73(3):540–545
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
Yang JK, Barnett MO, Jardine PM, Basta NT, Casteel SW (2002) Adsorption, sequestration, and bioaccessibility of As(V) in soils. Environ Sci Technol 36:4562–4569
Zhang H, Selim MH (2008) Competitive sorption-desorption kinetics of arsenate and phosphate in soils. Soil Sci 173(1):3–12
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashem, M.A., Toda, K. & Ohira, SI. Leaching behavior of arsenite and arsenate from the contaminated sediment by the effect of phosphate ion under anaerobic conditions. Environ Earth Sci 74, 737–743 (2015). https://doi.org/10.1007/s12665-015-4078-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4078-3