Abstract
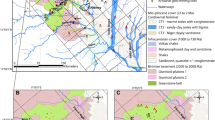
The Gilt Edge Superfund Site is a former heap-leach gold mine that currently is being remediated in the Black Hills of South Dakota. Mine runoff water is treated before release from the site. The field pH, before treatment, is about 3; the water contains arsenic at low levels and some trace metals at elevated levels, in addition to total dissolved solids concentrations of more than 1,900 mg/L. In the Keystone area of the Black Hills, naturally occurring arsenic has been detected at elevated concentrations in groundwater samples from wells. The City of Keystone’s Roy Street Well, which is not used currently, showed arsenic concentrations of 36 parts per billion and total dissolved solids of 320 mg/L. With field samples of water from the Gilt Edge site, a limestone-based method was successful in reducing trace metals concentrations to about 0.001 mg/L or less; at the Keystone site, the limestone method reduced arsenic levels to about 0.006 mg/L. The results are significant because previous research with the limestone-based method mainly had involved samples prepared with distilled water in the laboratory, in which interference of other ions such as sulfate did not occur. The research indicates the potential for broader applications of the limestone-based removal method, including scale-up work at field sites for water treatment.





Similar content being viewed by others
References
Cherry JA, Shaikh AU, Tallman DE, Nicholson RV (1979) Arsenic species as an indication of redox conditions in groundwater. J Hydrol 43:373–392
Chintalapati PK, Davis AD, Hansen MR, Sorensen JL, Dixon DJ (2009) Encapsulation of limestone waste in concrete after arsenic removal from drinking water. Environ Earth Sci 59(1):185–190
Davis AD, Webb CJ, Durkin TV (1999) A watershed approach to evaluating impacts of abandoned mines in the Bear Butte Creek basin of the Black Hills. Min Eng 51(9):49–56
Davis AD, Webb CJ, Dixon DJ, Sorensen JL, Dawadi S (2007) Arsenic removal from drinking water by limestone-based material. Min Eng 59(2):71–74
Drever JI (1997) The geochemistry of natural waters, 3rd edn. Prentice-Hall, Upper Saddle River, p 436
Fivecoate RI (2004) Characterization of limestone and dolomite used in the removal of arsenic and heavy metals from water. M.S. Thesis, South Dakota School of Mines and Technology, Rapid City, SD: 61 p
Juillot F, Ildefonse Ph, Morin G, Calas G, de Kersabiec AM, Benedetti M (1999) Remobilization of arsenic from buried wastes at an industrial site: mineralogical and geochemical control. Appl Geochem 14(8):1031–1048
Powell JE, Norton JJ, Adolphson DG (1973) Water resources and geology of Mount Rushmore National Memorial, South Dakota. US Geol Surv Water Supply Pap 1865:50
Sorensen JL (2007) Controlling factors on arsenic removal from water by limestone-based materials. Ph.D. dissertation, South Dakota School of Mines and Technology, Rapid City, South Dakota, p 284
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davis, A.D., Webb, C.J., Sorensen, J.L. et al. Laboratory testing of trace metals removal from mine drainage and arsenic removal from groundwater in the Black Hills of South Dakota. Environ Earth Sci 72, 355–361 (2014). https://doi.org/10.1007/s12665-013-2956-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2956-0