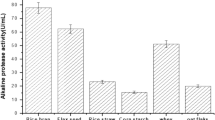
Abstract
The catalytic depolymerization of biological enzymes during biological liquefaction of coal is very important. In this study, the effects of medium types, lignite addition, and mixed culture on lignin peroxidase (LiP), manganese peroxidase (MnP) and laccase (Lac) secreted by white-rot fungi are investigated. Fifteen strains of white-rot fungi are used for microbial liquefaction of the Zhaotong lignite in Yunnan. The results show that the activities of LiP, MnP, and Lac are highest in the Czapek-Dox medium (DOX), followed by the Sabouraud Maltose Broth (SMB) medium, and are almost zero in the Potato Dextrose Agar (PDA) medium. In the early stage of fungal growth, the medium contains sufficient nutrients, and lignite addition induces an inhibitory effect on extracellular enzyme secretion, whereas in the late growth stage characterized by nutrient deficiency, it exhibits an enhancing effect. For fungi pair combinations, if only single enzyme activity was considered, the positive synergistic effect of Lac in the mixed culture of s.commune and SL203 would be the highest, with the synergistic coefficient of 233.33. While three enzymes were considered, the integrated effects of ZTW07 and SL203 are the highest, with Lac, MnP, and LiP producing synergistic coefficients of 158.795, 1.217, and 1.006, respectively. It can be seen that the mixed culture can greatly improve the enzyme activity of white rot fungi and is one of the effective methods to improve the liquefaction efficiency.
Graphic Abstract








Similar content being viewed by others
References
Hölker, U., Ludwig, S., Scheel, T., Höfer, M.: Mechanisms of coal solubilization by the deuteromycetes Trichoderma atroviride and Fusarium oxysporum. Appl. Microbiol. Biotechnol. 52(1), 57–59 (1999)
Hayatsu, R., Winans, R.E., Mcbeth, R.L., Ssott, R.G., Moore, L.P., Studier, M.H.: Lignin-like polymers in coals. Nature 78, 41–43 (1979)
Vamvuka, D., Sfakiotakis, S.: Combustion behaviour of biomass fuels and their blends with lignite. Thermochim. Acta 526(1–2), 192–199 (2011)
Küçük, A., Kadıoğlu, Y., Gülaboğlu, M.Ş.: A study of spontaneous combustion characteristics of a turkish lignite: particle size, moisture of coal, humidity of air. Combust. Flame 133(3), 255–261 (2003)
Wang, J., Fan, W., Li, Y., Xiao, M., Wang, K., Ren, P.: The effect of air staged combustion on NOx emissions in dried lignite combustion. Energy 37(1), 725–736 (2012)
Stefanova, M., Marinov, S.P., Mastral, A.M., Callén, M.S., Garci, T.: Emission of oxygen, sulphur and nitrogen containing heterocyclic polyaromatic compounds from lignite combustion. Fuel Process. Technol. 77–78, 89–94 (2002)
Butuzova, L., Krzton, A., Bazarova, O.: Structure and properties of humic acids obtained from thermo-oxidised brown coal. Fuel 77(6), 581–584 (1998)
Cohen, M.S., Gabriele, P.D.: Degradation of coal by the fungi Polyporus Versicolor and Poria monticola. Appl. Environ. Microbiol. 44(1), 23–27 (1982)
Crawford, D.L., Gupta, R.K.: Influence of cultural parameters on the depolymerization of a soluble lignite coal polymer by Pseudomonas cepacia DLC-07. Resour. Conserv. Recycl. 5(2–3), 245–254 (1991)
Quigley, D., Ward, B., Crawford, D., et al.: Evidence that microbially produced alkaline materials are involved in coal biosolubilization. Appl. Biochem. Biotechnol. 20–21(1), 753–763 (1989)
Strandbrg, G.W., Lewis, S.N.: Solubilization of coal by an extracellular product from Streptomyces setonii 75Vi2. J. Ind. Microbiol. Biotechnol. 1(6), 371–375 (1987)
Bublitz, F., Gunther, T., Frische, W.: Screening of fungi for the biological modification of hard coal and coal derivatives. Fuel Process. Technol. 40(2-3), 347–354 (1994)
David, Y., Baylon, M.G., Pamidimarri, S.D., Baritugo, K.A., Chae, C.G., Kim, Y.J., Kim, T.W., Kim, M.S., et al.: Screening of microorganisms able to degrade low-rank coal in aerobic conditions: potential coal biosolubilization mediators from coal to biochemicals. Biotechnol. Bioprocess Eng. 22, 178–185 (2017)
Li, J., Liu, X., Yue, Z., et al.: Biodegradation of photo- oxidized lignite and characterization of the products. IOP Conf. Ser. 108, 042122 (2018)
Haider, R., Ghauri, M.A., Akhter, K.: Isolation of coal degrading fungus from drilled core coal sample and effectof prior fungal pretreatment on chemical attributes of extracted humic acid. Geomicrobiol. J. 32(10), 944–953 (2015)
Silva-Stenico, E., Vengadajellum, C.J., Janjua, H.A., Harrison, S.T.L., Burton, S.G., Cowan, D.A.: Degradation of low rank coal by Trichoderma atroviride ES11. J. Indus. Microbiol. Biotechnol. 34(9), 625–631 (2007)
Haider, R., Ghauri, M.A., SanFilipo, J.R., Jones, E.J., Orem, W.H., Tatu, C.A., Akhtar, K., Akhtar, N.: Fungal degradation of coal as a pretreatment for methane production. Fuel 104, 717–725 (2013)
Ralph, J.P., Catcheside, D.E.A.: Transformations of low rank coal by Phanerochaete chrysosporium andother wood-rot fungi. Fuel Process. Technol. 52(1–3), 79–93 (1997)
Tien, M., Kirk, T.K.: Lignin degrading enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science 221(4611), 661–663 (1983)
Cohen, M.S., Bowers, W.C., Aronson, H., Gray Jr, E.T.: Cell-free solubilization of coal by Polyporus Versicolor. Appl. Environ. Microbiol. 53(12), 2840–2843 (1987)
Ralph, J.P., Catcheside, D.E.A.: Decolourisation and depolymerisation of solubilised low-rank coal by the white-rot basidiomycete Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 42(4), 536–542 (1994)
Shi, K., Yin, S., Tao, X., Du, Y., He, H., Lv, Z., Xu, N.: Quantitative measurement of coal bio-solubilization by ultraviolet-visible spectroscopy. Energy Sources Part A 35(15), 1456–1462 (2013)
Fakoussa, R.M., Hofrichter, M.: Biotechnology and microbiology of coal degradation. Appl. Microbiol. Biotechnol. 52(1), 25–40 (1999)
Glenn, J.K., Morgan, M.A., Mayfield, M.B., Kuwahara, M., Gold, M.H.: An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem. Biophys. Res. Commun. 114(3), 1077–1083 (1983)
Hammel, K.E., Cullen, D.: Role of fungal peroxidases in biological ligninolysis. Curr. Opin. Plant Biol. 11(3), 349–355 (2008)
Asgher, M., Shah, S.A.H., Ali, M., Legge, R.L.: Decolorization of some reactive textile dyes by white rot fungi isolated in Pakistan. World J. Microbiol. Biotechnol. 22(1), 89–93 (2006)
MingTien, T., Kirk, K.: Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol. 161, 238–249 (1988)
Haemmerli, S.D., Leisola, M.S., Sanglard, D., Fiechter, A.: Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J. Biol. Chem. 261, 6900–6903 (1986)
Hammel, K.E., Kalyanaraman, B., Kirk, T.K.: Oxidation of polycyclic aromatic hydrocarbons and dibenzo[p]-dioxins by Phanerochaete chrysosporium ligninase. J. Biol. Chem. 261, 16948–16952 (1986)
Paszczynski, A., Huynh, V.B., Crawford, R.: Enzymatic activities of an extracellular, manganese-dependent peroxidase from Phanerochaete chrysosporium. FEMS Microbiol. Lett. 29(1–2), 37–41 (1985)
Brown, J.A., Alic, M., Gold, M.H.: Manganese peroxidase gene transcription in Phanerochaete chrysosporium: activation by manganese. J. Bacteriol 173(13), 4101–4106 (1991)
Hofrichter, M.: Review: lignin conversion by manganese peroxidase (MnP). Enzyme Microbial. Technol. 30(4), 454–466 (2002)
Hofrichter, M., Scheibner, K., Bublitz, F., Schneegaß, I., Ziegenhagen, D., Martens, R., Fritsche, W.: Depolymerization of straw lignin by manganese peroxidase from Nematoloma frowardii is accompanied by release of carbon dioxide. Holzforschung 53(2), 161–166 (1999)
Hikorokuro, Y.: Chemistry of lacquer (Urushi). Part I. Communication from the Chemical Society of Tokio. J. Chem. Soc. Trans. 43, 472–486 (1883)
Claus, H.: Laccases: structure, reactions, distribution. Micron 35(1–2), 93–96 (2004)
Hoegger, P.J., Kilaru, S., James, T.Y., Thachker, J.R., Kues, U.: Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J. 273(10), 2308–2326 (2006)
Bertrand, T., Jolivalt, C., Briozzo, P., Caminade, E., Joly, N., Madzak, C., Mougin, C.: Crystal structure of four-copper laccase complexed with an arylamide: insights into substrate recognition and correlation with kinetics. Biochemistry 41(23), 7325–7333 (2002)
Rodrı’guez, E., Pickard, M.A., Vazquez-Duhalt, R.: Industrial dye decolorization by Laccases from Ligninolytic Fungi. Curr. Mirobiol. 38, 27–32 (1999)
Dittmer, J.K., Patel, N.J., Dhawale, S.W., Dhawale, S.S.: Production of multiple laccase isoforms by Phanerochaete chrysosporium grown under nutrient sufficiency. FEMS Microbiol. Lett. 149(1), 65–70 (1997)
Pointek, K., Antorini, M., Choinowski, T.: Crystal structure of a laccase from the fungus Trametes versicolor at 190 Å resolution containing a full complement of coppers. J. Biol. Chem. 277(40), 37663–37669 (2002)
Kau, L.S., SpiraSolomon, D.J., Penner-Hahn, J.E., Hodgson, K.O., Solomon, E.I.: X-ray absorption edge determination of the oxidation stateand coordination number of copper: application to the Type3 site in Rhus vernicifera laccase and its reaction with oxygen. J. Am. Chem. Soc. 109, 6433–6442 (1987)
Morpirgo, L., Calabrese, L., Desideri, A., Rotilio, G.: Dependence on freezing of the geometry and redox potential of Type 1 and Type 2 copper sites of Japanese-lacquer-tree (Rhus vernic(fera) laccase. Biochem. J. 193, 639–642 (1982)
Witayakran, S., Ragauskas, A.J.: Synthetic applications of laccase in green chemistry. Adv. Synth. Catal. 351, 1187–1209 (2009)
Vasconcelos, A.F.D., Barbosa, A.M., Dekker, R.F.H., Scarminio, I.S., Rezende, M.I.: Optimization of laccase production by Botryosphaeria sp. in the presence of veratryl alcohol by the response-surface method. Process Biochem. 35(10), 1131–1138 (2000)
Arora, D.S., Chander, M., KaurGill, P.: Involvement of lignin peroxidase, manganese peroxidase and laccase in degradation and selective ligninolysis of wheat straw. Int. Biodeterior. Biodegradation 50(2), 115–120 (2001)
Erkurt, E.A., Ünyayar, A., Kumbur, H.: Decolorization of synthetic dyes by white rot fungi, involving laccase enzyme in the process. Process Biochem. 42(10), 1429–1435 (2007)
Stajić, M., Persky, L., Friesem, D., Hadar, Y., Wasser, S.P., Nevo, E., Vukojević, J.: Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species. Enzyme Microbial. Technol. 38(1–2), 65–73 (2006)
Archibald, F.S.: A new assay for lignin type peroxidases employing the dye Azure B. Appl. Environ. Microbiol. 58(9), 3110–3116 (1992)
Miller, R., Kuglin, J., Gallagher, G., Flurkey, W.H.: A spectrophotometric assay for laccase using o-tolidine. J. Food Biochem. 21(1), 445–459 (1997)
Fenice, M., Sermanni, G.G., Federici, F., D'Annibale, A.: Submerged and solid-state production of laccase and Mn-peroxidase by Panus tigrinus on olive mill wastewater-based media. J. Biotechnol. 100(1), 77–85 (2003)
Gokcay, C.F., Kolankaya, N., Dilek, F.B.: Microbial solubilization of lignites. Fuel 80, 1421–1433 (2001)
Bumpus, J.A., Senko, J., Lynd, G., Morgan, R., Sturm, K., Stimpson, J., Roe, S.: Biomimetic solubilization ofa low-rank coal: implications for use in methane production. Fuel Energy 12, 664–671 (1998)
Woodward, C.A., Kaufman, E.N.: Enzymatic catalysis in organic solvents: Polyethylene glycol modified hydrogenase retains sulfhydrogenase activity in toluene. Biotechnol. Bioeng. 52(3), 423–428 (1996)
Tao, X., Pan, L., Shi, K., Chen, H., Yin, S., Luo, Z.: Bio-solubilization of Chinese lignite I: extra-cellular protein analysis. Min. Sci. Technol. (China) 19(3), 358–362 (2009)
Zouari, H., Labat, M., Sayadi, S.: Degradation of 4-chlorophenol by the white rot fungus Phanerochaete chrysosporium in free and immobilized cultures. Bioresour. Technol. 84(2), 145–150 (2002)
Gumul, D., Ziobro, R., Noga, M., Sabat, R.: Characterisation of five potato cultivars according to their nutritional and pro-health components. Acta Sci. Polym. Technol. Aliment. 10(1), 77–81 (2011)
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 51504134), Key Supported Discipline of Guizhou Provence (Qian Xuewei He Zi ZDXK[2016]24), 2011 Collaborative Innovation Center of Guizhou Province (QianJiaohexietongchuangxinzi [2016]02), Guizhou Key Laboratory of Coal Clean Utilization (qiankehepingtairencai [2020]2001), and Liupanshui Key Laboratory of thermoelectric and electrode materials(52020-2020-0903).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, K., Liu, Y., Chen, P. et al. Contribution of Lignin Peroxidase, Manganese Peroxidase, and Laccase in Lignite Degradation by Mixed White-Rot Fungi. Waste Biomass Valor 12, 3753–3763 (2021). https://doi.org/10.1007/s12649-020-01275-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01275-z