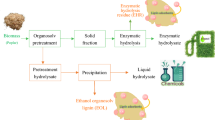
Abstract
Implementation of green chemistry and biorefinery concept are needed to boost production of biomass-derived fuels, chemicals, and materials with cost-effective processing of sustainable feedstock. The use of imidazole as a novel solvent for biomass pretreatment creates an approach that helps accomplish this concept. The present work is dedicated to study the pretreatment of residual lignocellulosic biomass, namely, extracted solid waste of Cupressus lusitanica, by application of the alkaline solvent—imidazole. The pretreatment allowed obtaining cellulose- and hemicellulose-rich fractions, whereas lignin was depolymerized. Both cellulose and hemicellulose recovery were highly dependent on the reaction conditions. The highest cellulose content was obtained at 160 °C for 4 h and was 40.7 ± 0.6 wt% with a delignification yield of 65.2 ± 0.4 wt%. The effect of biomass delignification on the efficiency of enzymatic digestibility was also analyzed and it was observed a good linear relationship between the delignification yield and the glucan to glucose yield. The presence of added-value phenolic compounds from depolymerized lignin in recovered imidazole was analyzed by capillary electrophoresis and determination of total phenolic content and antioxidant activity was also performed. These compounds were tentatively identified and their structures proposed on the basis of the HPLC–MS analyzes.
Graphic Abstract









Similar content being viewed by others
References
Silveira, M.H.L., Morais, A.R.C., da Costa Lopes, A.M., Olekszyszen, D.N., Bogel-Łukasik, R., Andreaus, J., Pereira, R.L.: Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. Chemsuschem 8(20), 3366–3390 (2015)
Magalhães da Silva, S.P., da Costa Lopes, A.M., Roseiro, L.B., Bogel-Łukasik, R.: Novel pre-treatment and fractionation method for lignocellulosic biomass using ionic liquids. RSC Adv. 3, 16040–16050 (2013). https://doi.org/10.1039/c3ra43091j
Hallett, J.P., Chambon, C.L., Chen, M., Fennell, P.S.: Efficient fractionation of lignin-and ash-rich agricultural residues following treatment with a low-cost protic ionic liquid. Front. Chem. 7, 246 (2019)
Mäki-Arvela, P., Anugwom, I., Virtanen, P., Sjoholm, R., Mikkola, J.P.: Dissolution of lignocellulosic materials and its constituents using ionic liquids: a review. Ind. Crops Prod. 32, 175–201 (2010). https://doi.org/10.1016/j.indcrop.2010.04.005
Bernardo, J.R., Gírio, F.M., Łukasik, R.M.: The effect of the chemical character of ionic liquids on biomass pre-treatment and posterior enzymatic hydrolysis. Molecules 24, 808 (2019). https://doi.org/10.3390/molecules24040808
Janesko, B.G.: Modeling interactions between lignocellulose and ionic liquids using DFT-D. Phys. Chem. Chem. Phys. 13, 11393–11401 (2011)
Kilpeläinen, I.A., Xie, H., King, A., Granstrom, M., Heikkinen, S., Argyropoulos, D.S.: Dissolution of wood in ionic liquids. J. Agric. Food Chem. 55, 9142–9148 (2007). https://doi.org/10.1021/jf071692e
Jordan, T., Schmidt, S., Liebert, T., Heinze, T.: Molten imidazole: a starch solvent. Green Chem. 16, 1967–1973 (2014)
Kang, Y., Realff, M.J., Sohn, M., Lee, J.H., Bommarius, A.S.: An Effective Chemical Pretreatment Method for Lignocellulosic Biomass with Substituted Imidazoles. Am. Inst. Chem. Eng. 31, 23–34 (2015)
Morais, A.R.C., Pinto, J.V., Nunes, D., Roseiro, L.B., Oliveira, M.C., Fortunato, E., Bogel-Łukasik, R.: Imidazole: prospect solvent for lignocellulosic biomass fractionation and delignification. ACS Sustain. Chem. Eng. 4, 1643–1652 (2016). https://doi.org/10.1021/acssuschemeng.5b01600
Toscan, A., Fontana, R.C., Andreaus, J., Camassola, M., Lukasik, R.M., Dillon, A.J.P.: New two-stage pretreatment for the fractionation of lignocellulosic components using hydrothermal pretreatment followed by imidazole delignification: focus on the polysaccharide valorization. Bioresour. Technol. 285, 121346 (2019)
Fockink, D.H., Andreaus, J., Ramos, L.P., Łukasik, R.M.: Pretreatment of cotton spinning residues for optimal enzymatic hydrolysis: a case study using green solvents. Renew. Energy. 145, 490–499 (2020). https://doi.org/10.1016/j.renene.2019.06.042
Toscan, A., Morais, A.R.C., Paixão, S.M., Alves, L., Andreaus, J., Camassola, M., Dillon, A.J.P., Lukasik, R.M.: Effective extraction of lignin from elephant grass using imidazole and its effect on enzymatic saccharification to produce fermentable sugars. Ind. Eng. Chem. Res. 56, 5138–5145 (2017). https://doi.org/10.1021/acs.iecr.6b04932
Sluiter, A., Hames, B., Hyman, D., Payne, C., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Wolfe, J.: Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples. National Renewable Energy Laboratory, Golden (2008)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D.: Determination of Structural Carbohydrates and Lignin in Biomass - Laboratory Analytical Procedure (LAP). National Renewable Energy Laboratory, Golden (2011)
Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D.: Determination of Extractives in Biomass. National Renewable Energy Laboratory, Golden (2008)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D.: Determination of Ash in Biomass. National Renewable Energy Laboratory, Golden (2008)
Milk and milk products - Determination of nitrogen content - Part 1: Kjeldahl principle and crude protein calculation. International Organization for Standarization (2014)
da Costa Lopes, A.M., João, K.G., Bogel-Łukasik, E., Roseiro, L.B., Bogel-Łukasik, R.: Pretreatment and fractionation of wheat straw using various ionic liquids. J. Agric. Food Chem. 61, 7874–7882 (2013). https://doi.org/10.1021/jf401980p
Selig, M., Weiss, N., Ji, Y.: Enzymatic Saccharification of Lignocellulosic Biomass: Laboratory Analytical Procedure (LAP): Issue Date, 3/21/2008. National Renewable Energy Laboratory - NREL, Colorado 80401-3393 (2008)
Roseiro, L.B., Tavares, C.S., Roseiro, J.C., Rauter, A.P.: Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): Optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind. Crops Prod. 44, 119–126 (2013)
Özbek, H.N., Fockink, D.H., Yanık, D.K., Göğüş, F., Lukasik, R.: The green biorefinery concept for the valorisation of pistachio shell by high-pressure CO2/H2O system. J. Clean. Prod. 196, 842–851 (2018). https://doi.org/10.1016/j.jclepro.2018.06.062
Kumar, L., Chandra, R., Chung, P.A., Saddler, J.: Can the same steam pretreatment conditions be used for most softwoods to achieve good, enzymatic hydrolysis and sugar yields? Bioresour. Technol. 101, 7827–7833 (2010)
Park, N., Kim, H.-Y., Koo, B.-W., Yeo, H., Choi, I.-G.: Organosolv pretreatment with various catalysts for enhancing enzymatic hydrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 101, 7046–7053 (2010)
Trinh, L.T.P., Lee, Y.J., Lee, J.-W., Lee, H.-J.: Characterization of ionic liquid pretreatment and the bioconversion of pretreated mixed softwood biomass. Biomass Bioenerg. 81, 1–8 (2015)
Sun, N., Rahman, M., Qin, Y., Maxim, M.L., Rodriguez, H., Rogers, R.D.: Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 11, 646–655 (2009)
Garrote, G., Dominguez, H., Parajo, J.C.: Autohydrolysis of corncob: study of non-isothermal operation for xylooligosaccharide production. J. Food Eng. 52, 211–218 (2002). https://doi.org/10.1016/S0260-8774(01)00108-X
Morais, A.R.C., Da Costa Lopes, A.M., Bogel-Łukasik, R.: Carbon dioxide in biomass processing: Contributions to the green biorefinery concept. Chem. Rev. 115, 3–27 (2015). https://doi.org/10.1021/cr500330z
da Costa Lopes, A.M., Lins, R.M.G., Rebelo, R.A., Lukasik, R.M.: Biorefinery approach for lignocellulosic biomass valorisation with acidic ionic liquid. Green Chem. 20, 4043–4057 (2018)
Morais, A.R.C., Matuchaki, M.D.D.J., Andreaus, J., Bogel-Lukasik, R.: A green and efficient approach to selective conversion of xylose and biomass hemicellulose into furfural in aqueous media using high-pressure CO2 as a sustainable catalyst. Green Chem. 18, 2985–2994 (2016). https://doi.org/10.1039/c6gc00043f
Pan, X.J., Xie, D., Yu, R.W., Lam, D., Saddler, J.N.: Pretreatment of lodgepole pine killed by mountain pine beetle using the ethanol organosolv process: Fractionation and process optimization. Ind. Eng. Chem. Res. 46, 2609–2617 (2007). https://doi.org/10.1021/Ie061576l
García, A., González Alriols, M., Spigno, G., Labidi, J.: Lignin as natural radical scavenger. Effect of the obtaining and purification processes on the antioxidant behaviour of lignin. Biochem. Eng. J. 67, 173–185 (2012). https://doi.org/10.1016/j.bej.2012.06.013
Aadil, K.R., Barapatre, A., Sahu, S., Jha, H., Tiwary, B.N.: Free radical scavenging activity and reducing power of Acacia nilotica wood lignin. Int. J. Biol. Macromol. 67, 220–227 (2014). https://doi.org/10.1016/j.ijbiomac.2014.03.040
dos Santos, P.S.B., Erdocia, X., Gatto, D.A., Labidi, J.: Characterisation of Kraft lignin separated by gradient acid precipitation. Ind. Crops Prod. 55, 149–154 (2014)
García, A., Toledano, A., Andrés, M.Á., Labidi, J.: Study of the antioxidant capacity of Miscanthus sinensis lignins. Process Biochem. 45, 935–940 (2010)
Vinardell, M.P., Ugartondo, V., Mitjans, M.: Potential applications of antioxidant lignins from different sources. Ind. Crops Prod. 27, 220–223 (2008)
Dizhbite, T., Telysheva, G., Jurkjane, V., Viesturs, U.: Characterization of the radical scavenging activity of lignins––natural antioxidants. Bioresour. Technol. 95, 309–317 (2004)
Ugartondo, V., Mitjans, M., Vinardell, M.P.: Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 99, 6683–6687 (2008)
Owen, B.C., Haupert, L.J., Jarrell, T.M., Marcum, C.L., Parsell, T.H., Abu-Omar, M.M., Bozell, J.J., Black, S.K., Kenttämaa, H.I.: High-performance liquid chromatography/high-resolution multiple stage tandem mass spectrometry using negative-ion-mode hydroxide-doped electrospray ionization for the characterization of lignin degradation products. Anal. Chem. 84, 6000–6007 (2012)
Banoub, J.H., Benjelloun-Mlayah, B., Ziarelli, F., Joly, N., Delmas, M.: Elucidation of the complex molecular structure of wheat straw lignin polymer by atmospheric pressure photoionization quadrupole time-of-flight tandem mass spectrometry. Rapid. Commun. Mass. Spectrom. 21, 2867–2888 (2007)
Morreel, K., Kim, H., Lu, F., Dima, O., Akiyama, T., Vanholme, R., Niculaes, C., Goeminne, G., Inze, D., Messens, E.: Mass spectrometry-based fragmentation as an identification tool in lignomics. Anal. Chem. 82, 8095–8105 (2010)
Acknowledgements
This research was done using Biomass and Bioenergy Research Infrastructure (BBRI)- LISBOA-01-0145-FEDER-022059, supported by Operational Program for Competitiveness and Internationalization (PORTUGAL2020), by Lisbon Portugal Regional Operational Program (Lisboa2020) and by North Portugal Regional Operational Program (Norte2020) under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). Furthermore, this research was supported by the Fundação para a Ciência e a Tecnologia (FCT, Portugal) through grant IF/00471/2015 (RML), ID/QUI/00100/2019 and (Lisboa-01-0145-FEDER-022125-IST/RNEM). The authors also wish to thank Maria do Céu Penedo and Belina Ribeiro (UB, LNEG) for assistance in the HPLC analyzes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pereira, P.M.A., Bernardo, J.R., Oliveira, M.C. et al. Depolymerization of Lignin from Extracted Solid Waste of Cupressus lusitanica Mill. Biomass Using Imidazole. Waste Biomass Valor 12, 1341–1355 (2021). https://doi.org/10.1007/s12649-020-01087-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01087-1